Top Previous Year Questions - Biomolecules
Question
Consider the following reactions :
(i) Glucose acetyl derivative
(ii) Glucose acetyl derivative
(iii) Glucose acetyl derivative '' , '' and '' in these reactions are respectively.
JEE Main 2020 (02 Sep Shift 1)
Options
- A: &
- B: &
- C: &
- D: &
Explaination
Question
The correct observation in the following reaction is:
JEE Main 2020 (02 Sep Shift 2)
Options
- A: Formation of blue colour
- B: Gives no colour
- C: Formation of red colour
- D: Formation of violet colour
Explaination
Question
The number of chiral centres present in threonine is _________
JEE Main 2020 (04 Sep Shift 2)
Enter your answer
Explaination
Question
The correct structure of Ruhemann's Purple, the compound formed in the reaction of Ninhydrin with proteins is:
JEE Main 2021 (20 Jul Shift 1)
Options
-
A:
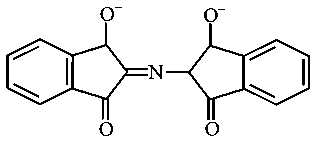
-
B:
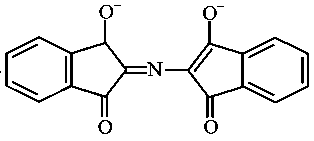
-
C:
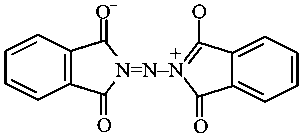
-
D:
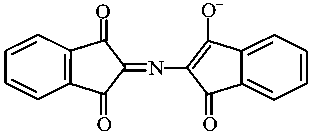
Explaination
Question
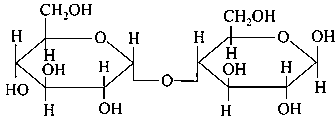
Which of the following is correct structure of -anomer of maltose?
JEE Main 2021 (25 Feb Shift 2)
Options
-
A:

-
B:
-
C:
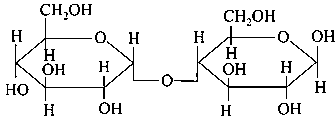
-
D:
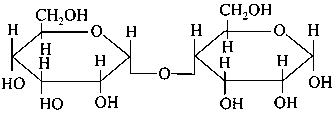
Explaination
Question
Hydrolysis of sucrose gives:
JEE Main 2021 (27 Aug Shift 2)
Options
- A: Glucose and -Fructose
- B: Glucose and Fructose
- C: -Glucose and -Fructose
- D: and -Fructose
Explaination
Question
Compound A gives D-Galactose and D-Glucose on hydrolysis. The compound A is :
JEE Main 2021 (27 Jul Shift 2)
Options
- A: Amylose
- B: Sucrose
- C: Maltose
- D: Lactose
Explaination
Question
A polysaccharide '' on boiling with dil at under pressure yields '' '' on treatment with bromine water gives gluconic acid. '' contains -glycosidic linkages only. Compound '' is :
JEE Main 2022 (24 Jun Shift 1)
Options
- A: starch
- B: cellulose
- C: amylose
- D: amylopectin
Explaination
Question
The formulas of and for the following reaction sequence are
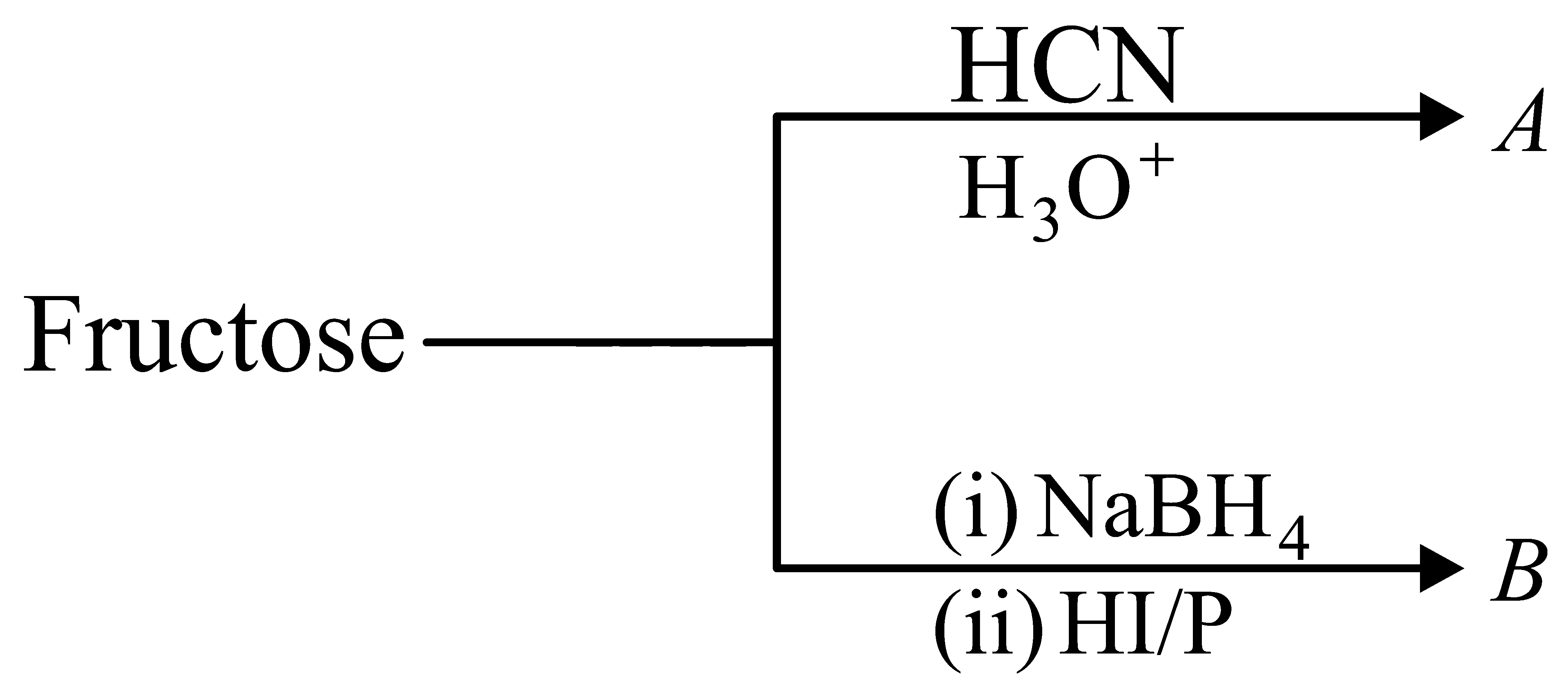
JEE Main 2022 (28 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
All structures given below are of vitamin . Most stable of them is:
JEE Main 2023 (01 Feb Shift 2)
Options
-
A:
-
B:
-
C:
-
D:
Explaination
Question
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): -halocarboxylic acid on reaction with dil. gives good yield of -amino carboxylic acid whereas the yield of amines is very low when prepared from alkyl halides.
Reason (R): Amino acids exist in zwitter ion form in aqueous medium.
In the light of the above statements, choose the correct answer from the options given below :
JEE Main 2023 (01 Feb Shift 2)
Options
- A: Both (A) and (R) are correct and (R) is the correct explanation of (A).
- B: Both (A) and (R) are correct but (R) is not the correct explanation of (A).
- C: (A) is correct but (R) is not correct.
- D: (A) is not correct but (R) is correct.
Explaination
Question
Match items of Row I with those of Row II.
Row I:
(P)
(Q)
(R)
(S)
Row II:
(i) Fructofuranose.
(ii) Fructofuranose.
(iii) Glucopyranose.
(iv) Glucopyranose.
Correct match is
JEE Main 2023 (25 Jan Shift 1)
Options
- A: P→iv, Q→iii, R→i, S→ii
- B: P→i, Q→ii, R→iii, S→iv
- C: P→iii, Q→iv, R→ii, S→i
- D: P→iii, Q→iv, R→i, S→ii
Explaination
Question
Following tetrapeptide can be represented as
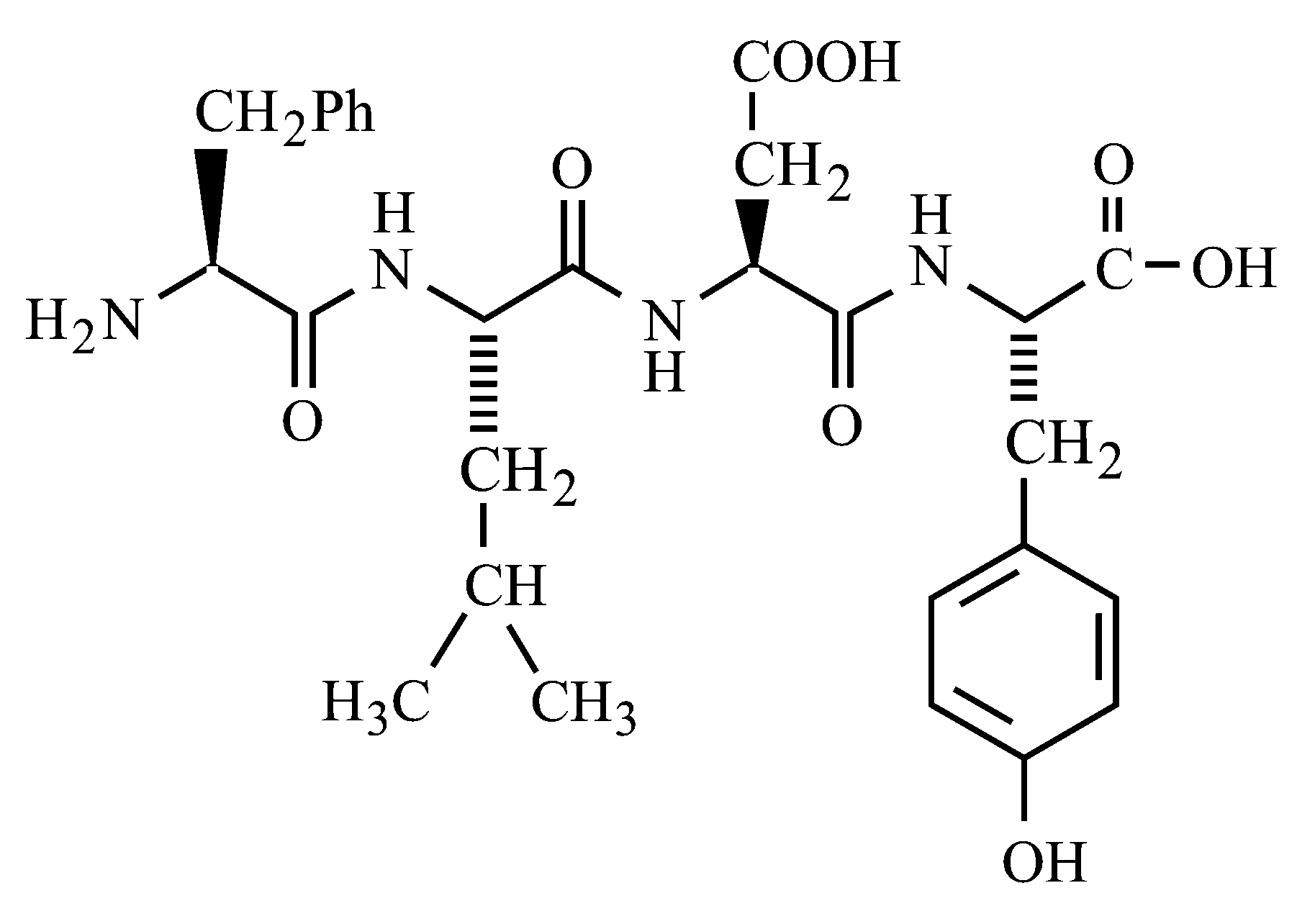
( are one letter codes for amino acids)
JEE Main 2023 (29 Jan Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): Ketoses give Seliwanoff’s test faster than Aldoses.
Reason (R): Ketoses undergo -elimination followed by formation of furfural.
In the light of the above statements, choose the correct answer from the options given below:
JEE Main 2023 (30 Jan Shift 1)
Options
- A: (A) is false but (R) is true
- B: Both (A) and (R) are true and (R) is the correct explanation of (A)
- C: (A) is true but (R) is false
- D: Both (A) and (R) are true but (R) is not the correct explanation of (A)
Explaination
Question
Compound , given a tetraacetate with and oxidation of with gives an acid, . Reduction of with gives isopentane. The possible structure of is :
JEE Main 2023 (31 Jan Shift 2)
Options
-
A:
-
B:
-
C:
-
D:
Explaination
Question
Which is not true for arginine?
JEE Main 2023 (15 Apr Shift 1)
Options
- A: It has a fairly high melting point
- B: It is associated with more than one values.
- C: It has high solubility in benzene.
- D: It is a crystalline solid.
Explaination
Question
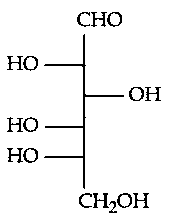
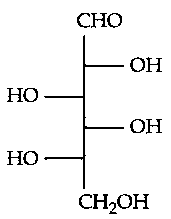
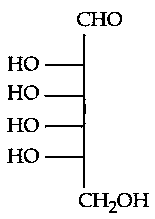
Which of the following is the correct structure of L-Glucose?
JEE Main 2024 (04 Apr Shift 1)
Options
-
A:
-
B:
-
C:
-
D:
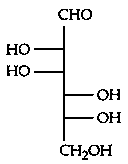
Explaination
Question
Match List I with List II \(\begin{array}{|l|l|c|l|} \hline & \text{List - I} & & \text{List - II} \\ \hline \text { A. } & \alpha \text { - Glucose and } \alpha \text { - Galactose } & \text { I. } & \text { Functional isomers } \\ \hline \text { B. } & \alpha \text { - Glucose and } \beta \text { - Glucose } & \text { II. } & \text { Homologous } \\ \hline \text { C. } & \alpha \text { - Glucose and } \alpha \text { - Fructose } & \text { III. } & \text { Anomers } \\ \hline \text { D. } & \alpha \text { - Glucose and } \alpha \text { - Ribose } & \text { IV. } & \text { Epimers } \\ \hline \end{array}\) Choose the correct answer from the options given below:
JEE Main 2024 (04 Apr Shift 2)
Options
- A: A-IV, B-III, C-I, D-II
- B: A-III, B-IV, C-I, D-II
- C: A-IV, B-III, C-II, D-I
- D: A-III, B-IV, C-II, D-I
Explaination
Question
The total number of carbon atoms present in tyrosine, an amino acid, is _______
JEE Main 2024 (08 Apr Shift 2)
Enter your answer
Explaination
Question
The incorrect statement about Glucose is :
JEE Main 2024 (09 Apr Shift 2)
Options
- A: Glucose is soluble in water because of having aldehyde functional group
- B: Glucose remains in multiple isomeric form in its aqueous solution
- C: Glucose is one of the monomer unit in sucrose
- D: Glucose is an aldohexose
Explaination
Question
Match List I with List II
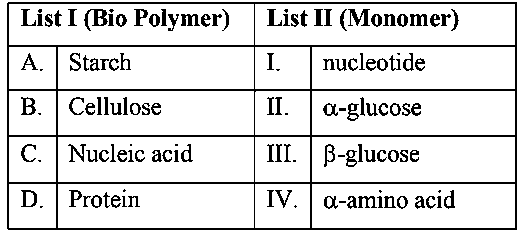 Choose the correct answer from the options given below :-
Choose the correct answer from the options given below :-
JEE Main 2024 (29 Jan Shift 2)
Options
- A: A-II, B-I, C-III, D-IV
- B: A-IV, B-II, C-I, D-III
- C: A-I, B-III, C-IV, D-II
- D: A-II, B-III, C-I, D-IV
Explaination
Question
Match List I with List I
| List I (Substances) | List II (Element Present) | ||
| A. | Ziegler catalyst | I. | Rhodium |
| B. | Blood Pigment | II. | Cobalt |
| C. | Wilkinson catalyst | III. | Iron |
| D. | Vitamin B12 | IV. | Titanium |
Choose the correct answer from the options given below:
JEE Main 2024 (29 Jan Shift 1)
Options
- A: A-II, B-IV, C-I, D-III
- B: A-II, B-III, C-IV, D-I
- C: A-III, B-II, C-IV, D-I
- D: A-IV, B-III, C-I, D-II
Explaination
Question
Match List I with List II
| List-I | List-II | ||
| A. | Glucose/ | I. | Gluconic acid |
| B. | Glucose/ | II. | No reaction |
| C. | Glucose/ | III. | -hexane |
| D. | Glucose/Bromine water | IV. | Saccharic acid |
Choose the correct answer from the options given below:
JEE Main 2024 (31 Jan Shift 1)
Options
- A: A-IV, B-I, C-III, D-II
- B: A-II, B-IV, C-III, D-I
- C: A-III, B-II, C-I, D-IV
- D: A-I, B-IV, C-III, D-II