Top Previous Year Questions - Chemical Bonding and Molecular Structure
Question
The correct statement among the following is:
JEE Main 2019 (12 Apr Shift 1)
Options
- A: is planar and less basic than
- B: is pyramidal and more basic than
- C: is pyramidal and less basic than
- D: is planar and more basic than
Explaination
Question
Of the species, and the one with minimum bond strength is :
JEE Main 2020 (03 Sep Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The potential energy curve for the molecule as a function of internuclear distance is:
JEE Main 2020 (05 Sep Shift 1)
Options
-
A:
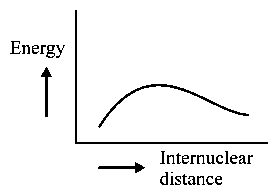
-
B:
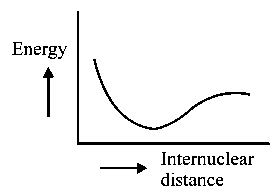
-
C:
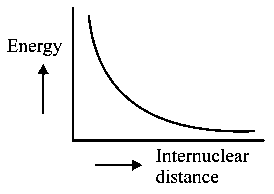
-
D:
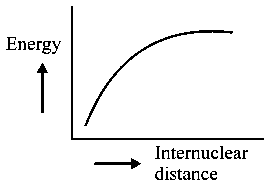
Explaination
Question
The increasing order of boiling points of the following compounds is :
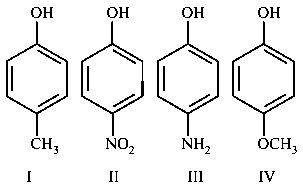
JEE Main 2020 (05 Sep Shift 2)
Options
- A: I < III < IV < II
- B: I < IV < II< III
- C: IV < I < II < III
- D: III < I < II < IV
Explaination
Question
If the magnetic moment of a di-oxygen species is , it may be
JEE Main 2020 (09 Jan Shift 1)
Options
- A: or .
- B: or .
- C: or .
- D: or .
Explaination
Question
Which of the following are isostructural pairs?
A. and
B. and
C. and
D. and
JEE Main 2021 (24 Feb Shift 1)
Options
- A: and only
- B: and only
- C: and only
- D: and only
Explaination
Question
The number of species below that have two lone pairs of electrons in their central atom is ___ (Round off to the Nearest integer)
JEE Main 2021 (18 Mar Shift 2)
Enter your answer
Explaination
Question
The correct set from the following in which both pairs are in correct order of melting point is:
JEE Main 2021 (24 Feb Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Match List I with List II
| List-I | List-II | ||
| A | I | ; linear | |
| B | II | ; pyramidal | |
| C | III | ; distorted octahedral | |
| D | IV | ; square pyramidal |
Choose the correct answer from the options given below
JEE Main 2022 (25 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Number of electron deficient molecules among the following and is
JEE Main 2022 (25 Jun Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Match List - I with List - II.
| List-I (Compound) | List-II (Shape) | ||
| A | I | bent | |
| B | II | square pyramidal | |
| C | III | trigonal bipyramidal | |
| D | IV | octahedral |
Choose the correct answer from the options given below
JEE Main 2022 (26 Jul Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Match List-I with List-II
| List-I | List-II | ||
| A | I | Dipole moment | |
| B | II | Bonding molecular orbital | |
| C | III | Anti-bonding molecualr orbital | |
| D | IV | Bond order |
JEE Main 2022 (27 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Consider the species and . Choose the correct option with respect to the there species:
JEE Main 2022 (29 Jun Shift 2)
Options
-
A: They are isoelectronic and only two have tetrahedral structures
-
B: They are isoelectronic and all have tetrahedral structures
-
C: Only two are isoelectronic and all have tetrahedral structures
- D: Only two are isoelectronic and only two have tetrahedral structures
Explaination
Question
Among the following species the number of species showing diamagnetism is
JEE Main 2022 (25 Jul Shift 1)
Enter your answer
Explaination
Question
Amongst the following the number of oxide(s) which are paramagnetic in nature is
JEE Main 2022 (27 Jul Shift 1)
Enter your answer
Explaination
Question
Consider, and
Amongst the above molecule(s) ion(s), the number of molecule(s)/ion(s) having hybridisation is
JEE Main 2022 (29 Jul Shift 2)
Enter your answer
Explaination
Question
The bond order and magnetic property of acetylide ion are same as that of
JEE Main 2023 (12 Apr Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Among the following compounds, the one which shows highest dipole moment is
JEE Main 2023 (13 Apr Shift 1)
Options
-
A:
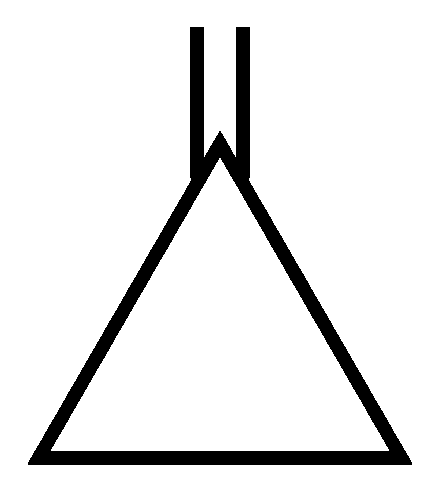
-
B:
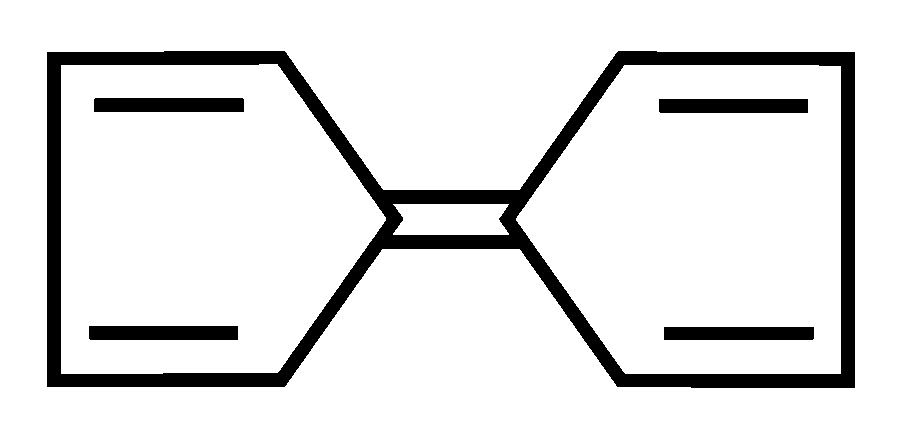
-
C:
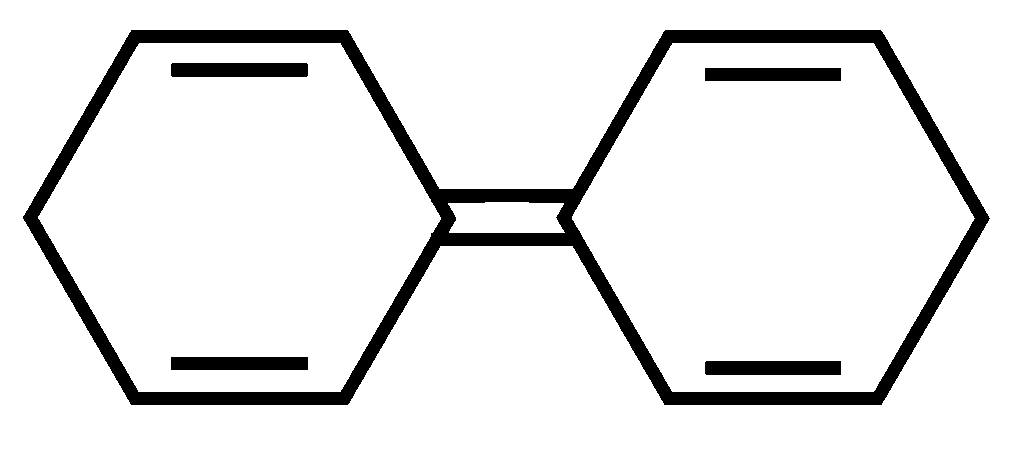
-
D:
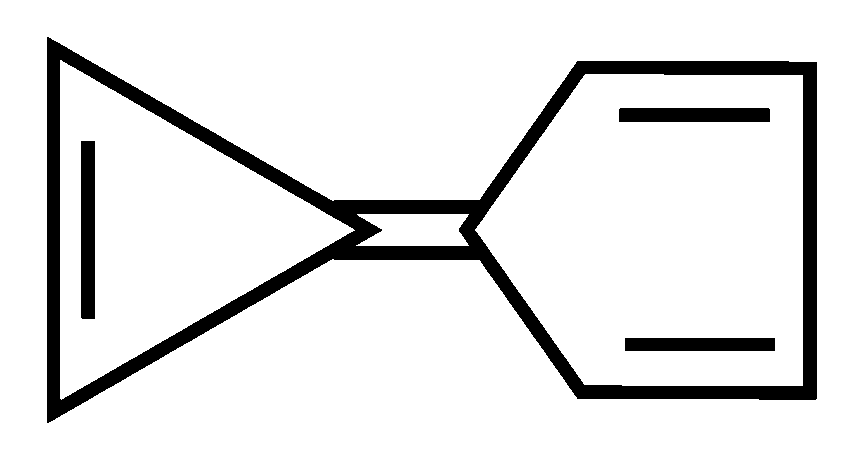
Explaination
Question
at room temperature is a
JEE Main 2023 (13 Apr Shift 1)
Options
-
A:
Colourless liquid with trigonal bipyramidal geometry
-
B:
Colourless gas with square pyramidal geometry
-
C:
Colourless gas with trigonal bipyramidal geometry
-
D:
Colourless liquid with square pyramidal geometry
Explaination
Question
Match Lis-I with List-II.
| List-1 | List-II | ||
| A. | Weak intermolecular forces of attraction | I. | Hexamethylenediamine + adipic acid |
| B. | Hydrogen bonding | II. | |
| C. | Heavily branched polymer | III. | |
| D. | High density polymer | IV. |
Choose the correct answer from the options given below
JEE Main 2023 (13 Apr Shift 2)
Options
- A: A-IV, B-II, C-III, D-I
- B: A-IV, B-I, C-III, D-II
- C: A-II, B-IV, C-I, D-III
- D: A-III, B-I, C-IV, D-II
Explaination
Question
Which one of the following molecules has maximum dipole moment?
JEE Main 2024 (04 Apr Shift 1)
Options
- A: \(\mathrm{NF}_3\)
- B: \(\mathrm{CH}_4\)
- C: \(\mathrm{PF}_5\)
- D: \(\mathrm{NH}_3\)
Explaination
Question
Number of molecules/ions from the following in which the central atom is involved in \(\mathrm{sp}^3\) hybridization is \(\qquad\) \(\mathrm{NO}_3^{-}, \mathrm{BCl}_3, \mathrm{ClO}_2^{-}, \mathrm{ClO}_3\)
JEE Main 2024 (04 Apr Shift 1)
Options
- A: 4
- B: 3
- C: 2
- D: 1
Explaination
Question
The correct statement/s about Hydrogen bonding is/are A. Hydrogen bonding exists when \(\mathrm{H}\) is covalently bonded to the highly electro negative atom. B. Intermolecular \(\mathrm{H}\) bonding is present in o-nitro phenol C. Intramolecular \(\mathrm{H}\) bonding is present in HF. D. The magnitude of \(\mathrm{H}\) bonding depends on the physical state of the compound. E. H-bonding has powerful effect on the structure and properties of compounds Choose the correct answer from the options given below:
JEE Main 2024 (04 Apr Shift 2)
Options
- A: A, B, D only
- B: A, D, E only
- C: A only
- D: A, B, C only
Explaination
Question
The number of species from the following that have pyramidal geometry around the central atom is _______. \(\mathrm{S}_2 \mathrm{O}_3^{2-}, \mathrm{SO}_4^{2-}, \mathrm{SO}_3^{2-}, \mathrm{S}_2 \mathrm{O}_7^{2-}\)
JEE Main 2024 (04 Apr Shift 2)
Options
- A: 4
- B: 3
- C: 2
- D: 1
Explaination
Question
Match List I with List II \(\begin{array}{llll} \text{List - I} & \text{List - II} \\ \text{(A) } \mathrm{ICl} & \text{(I) T - shape} \\ \text{(B) } \mathrm{ICl}_3 & \text{(II) pyramidal} \\ \text{(C) } \mathrm{ClF}_5 & \text{(III) Pentagonal bipyramidal} \\ \text{(D) } \mathrm{IF}_7 & \text{(IV) Linear} \\ \end{array}\) Choose the correct answer from the options given below :
JEE Main 2024 (05 Apr Shift 2)
Options
- A: (A)-(IV), (B)-(I), (C)-(II), (D)-(III)
- B: (A)-(I), (B)-(IV), (C)-(III), (D)-(II)
- C: (A)-(IV), (B)-(III), (C)-(II), (D)-(I)
- D: (A)-(I), (B)-(III), (C)-(II), (D)-(IV)