Top Previous Year Questions - Chemical Kinetics
Question
The rate constant of a reaction is measured at different temperature , and the data are plotted in the given figure. the activation energy of the reaction in is : is gas constant)

JEE Main 2020 (05 Sep Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Consider the following reactions
The order of the above reactions are respectively. The following graph is obtained when log[rate] vs.log[conc.] are plotted:

Among the following, the correct sequence for the order of the reactions is :
JEE Main 2020 (06 Sep Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The rate of a reaction decreased by times when the temperature was changed from . the activation energy (in ) of the reaction is ________ .
JEE Main 2020 (06 Sep Shift 2)
Enter your answer
Explaination
Question
Which one of the following given graphs represents the variation of rate constant with temperature for an endothermic reaction?
JEE Main 2021 (01 Sep Shift 2)
Options
-
A:
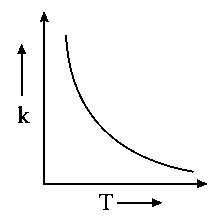
-
B:
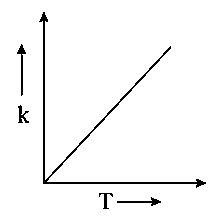
-
C:
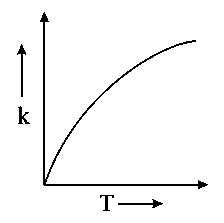
-
D:
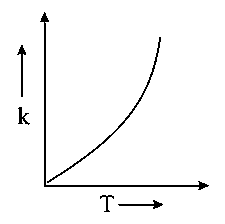
Explaination
Question
For the following graphs,
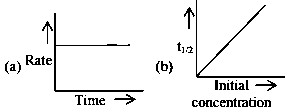
Choose from the options given below, the correct one regarding order of reaction is :
JEE Main 2021 (25 Jul Shift 1)
Options
- A: (b) zero order (c) and (e) First order
- B: (a) and (b) Zero order (e) First order
- C: (b) and (d) Zero order (e) First order
- D: (a) and (b) Zero order (c) and (e) First order
Explaination
Question
The decomposition of formic acid on gold surface follows first order kinetics. If the rate constant at is and the activation energy , the rate constant at is _________ . (Round of to the Nearest Integer).
(Given )
JEE Main 2021 (16 Mar Shift 1)
Enter your answer
Explaination
Question
This reaction was studied at and the following data was obtained
and are the initial concentrations and is the initial reaction rate. The overall order of the reaction is
(Round off to the Nearest Integer).
JEE Main 2021 (18 Mar Shift 1)
Enter your answer
Explaination
Question
A reaction has a half life of . The time required for completion of the reaction is ___ min. (Round off to the Nearest integer)
[Use : ]
JEE Main 2021 (18 Mar Shift 2)
Enter your answer
Explaination
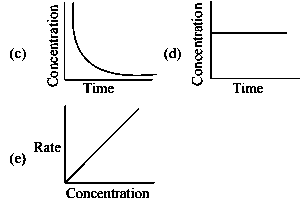
Question
For the reaction, the plot of v/s is given below:
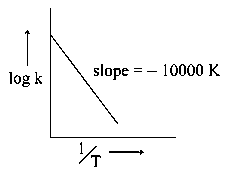
The temperature at which the rate constant of the reaction is is _____
(Rounded-off to the nearest integer) [Given : The rat5e constant of the reaction is at .]
JEE Main 2021 (25 Feb Shift 1)
Enter your answer
Explaination
Question
The following data was obtained for chemical reaction given below at .
The order of the reaction with respect to _____ is [Integer answer]
JEE Main 2021 (26 Aug Shift 1)
Enter your answer
Explaination
Question
The rate constants for decomposition of acetaldehyde have been measured over the temperature range . The data has been analysed by plotting vs graph. The value of activation energy for the reaction is . (Nearest integer) (Given : )
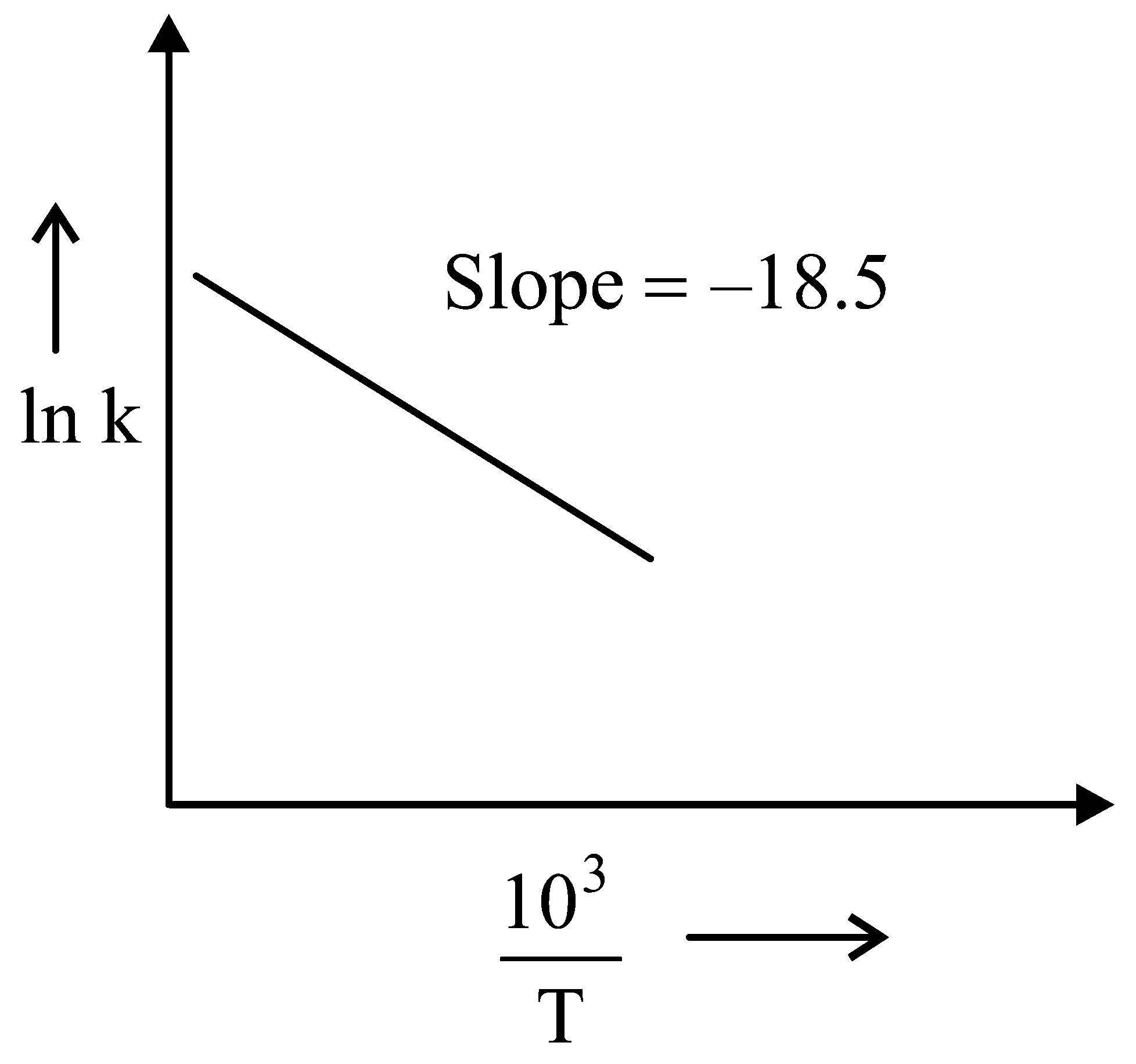
JEE Main 2022 (24 Jun Shift 1)
Enter your answer
Explaination
Question
The half life for the decomposition of gaseous compound is when the gaseous pressure was Torr initially. When the pressure was Torr, the half life was found to be . The order of the reaction is _____ (Nearest integer)
JEE Main 2022 (25 Jul Shift 1)
Enter your answer
Explaination
Question
The reaction between and is first order with respect to and zero order with respect to .
| Experiment | |||
| I | |||
| II | |||
| III | |||
| IV |
Examine the data of table and calculate ratio of numerical values of and .
JEE Main 2022 (29 Jul Shift 1)
Enter your answer
Explaination
Question
An organic compound undergoes first order decomposition. If the time taken for the decomposition is , then the time required for decomposition will be is _____ s. (Nearest integer).
Given :
JEE Main 2023 (30 Jan Shift 2)
Enter your answer
Explaination
Question
For the adsorption of hydrogen on platinum, the activation energy is and for the adsorption of hydrogen on nickel, the activation energy is The logarithm of the ratio of the rates of chemisorption on equal areas of the metals at is (Nearest integer)
Given:
JEE Main 2023 (06 Apr Shift 1)
Enter your answer
Explaination
Question
Consider the following reaction, the rate expression of which is given below \[ \begin{aligned} & \mathrm{A}+\mathrm{B} \rightarrow \mathrm{C} \\ & \text { rate }=\mathrm{k}[\mathrm{A}]^{1 / 2}[\mathrm{~B}]^{1 / 2} \end{aligned} \] The reaction is initiated by taking \(1 \mathrm{M}\) concentration of \(\mathrm{A}\) and \(\mathrm{B}\) each. If the rate constant \((\mathrm{k})\) is \(4.6 \times 10^{-2} \mathrm{~s}^{-1}\), then the time taken for \(\mathrm{A}\) to become \(0.1 \mathrm{M}\) is ______ sec. (nearest integer)
JEE Main 2024 (04 Apr Shift 2)
Enter your answer
Explaination
Question
During Kinetic study of reaction \(2 A+B \rightarrow C+D\), the following results were obtained : \(\begin{array}{llll} & \text { A [M] } & \text { B [M] } & \text { initial rate of formation of D } \\ \text { I } & 0.1 & 0.1 & 6.0 \times 10^{-3} \\ \text { II } & 0.3 & 0.2 & 7.20 \times 10^{-2} \\ \text { III } & 0.3 & 0.4 & 2.88 \times 10^{-1} \\ \text { IV } & 0.4 & 0.1 & 2.40 \times 10^{-2} \\ \end{array}\) Based on above data, overall order of the reaction is ______
JEE Main 2024 (05 Apr Shift 1)
Enter your answer
Explaination
Question
Consider the following reaction \[ \mathrm{A}+\mathrm{B} \rightarrow \mathrm{C} \] The time taken for \(\mathrm{A}\) to become \(1 / 4^{\text {th }}\) of its initial concentration is twice the time taken to become \(1 / 2\) of the same. Also, when the change of concentration of \(B\) is plotted against time, the resulting graph gives a straight line with a negative slope and a positive intercept on the concentration axis. The overall order of the reaction is _______
JEE Main 2024 (08 Apr Shift 1)
Enter your answer
Explaination
Question
required for a reaction is produced by decomposition of in as by equation
The initial concentration of is and it is after minutes.
The rate of formation of is , value of is ________.
JEE Main 2024 (30 Jan Shift 2)
Enter your answer
Explaination
Question
for a reaction, of is decomposed in minutes. The time taken for decomposition of is ______ minutes.
JEE Main 2024 (31 Jan Shift 2)
Enter your answer
Explaination
Question
The following data were obtained during the first order thermal decomposition of a gas at constant volume:
S. No Time/s Total pressure/(atm)
The rate constant of the reaction is _______ (nearest integer)
JEE Main 2024 (01 Feb Shift 2)
Enter your answer
Explaination
Question
Time required for completion of of first order reaction is ________ times of half life of the reaction
JEE Main 2024 (27 Jan Shift 2)
Enter your answer
Explaination
Question
The rate of first order reaction is at minutes and at minutes after initiation. Half life of the reaction is ______ minutes. (Given
Round off your answer to the nearest integer.
JEE Main 2024 (30 Jan Shift 1)
Enter your answer
Explaination
Question
Integrated rate law equation for a first order gas phase reaction is given by (where is initial pressure and is total pressure at time )
JEE Main 2024 (31 Jan Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Consider the following data for the given reaction
Rate
The order of the reaction is __________.