Top Previous Year Questions - Classification of Elements and Periodicity in Properties
Question
The correct order of the ionic radii of and is :
JEE Main 2020 (05 Sep Shift 2)
Options
- A:
- B:
- C:
-
D:
Explaination
Question
has a smaller first ionization enthalpy than . Consider the following statement:
(I) it is easier to remove electron than electron
(II) electron of is more shielded from the nucleus by the inner core of electrons than the electrons of
(III) electron has more penetration power than electron
(IV) atomic radius of is more than
(atomic number )
The correct statements are.
JEE Main 2020 (09 Jan Shift 1)
Options
- A: (I), (II) and (IV)
- B: (II), (III) and (IV)
- C: (I), (II) and (III)
- D: (I), (III) and (IV)
Explaination
Question
The set in which compounds have different nature is:
JEE Main 2021 (20 Jul Shift 1)
Options
- A: and
- B: and
- C: and
- D: and
Explaination
Question
The ionic radii of and respectively are and , while the covalent radius of is
The correct statement for the ionic radius of from the following is :
JEE Main 2021 (25 Jul Shift 2)
Options
- A: It is smaller than and
- B: It is bigger than and
- C: It is bigger than and , but smaller than of
-
D: It is smaller than and , but bigger than of
Explaination
Question
Match List- with List-.
| List- Electronic configuration of elements | List- in | ||
Choose the most appropriate answer from the options given below:
JEE Main 2021 (26 Feb Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Metallic character decreases and non-metallic character increases on moving from left to right in a period.
Reason (R): It is due to increase in ionisation enthalpy and decrease in electron gain enthalpy, when one moves from left to right in a period.
In the light of the above statements, choose the most appropriate answer from the options given below :
JEE Main 2021 (31 Aug Shift 1)
Options
- A: (A) is false but is true.
- B: Both and are correct and is the correct explanation of
- C: Both and are correct but is not the correct explanation of .
- D: is true but is false.
Explaination
Question
What is the correct oder of electron gain enthalpy of
JEE Main 2022 (25 Jun Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Element "" belongs to the period and group of the periodic table. The valence shell electron configuration of the element, which is just above "" in the group is
JEE Main 2022 (28 Jun Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The first ionization enthalpy of and , respectively, are: and . The first ionization enthalpy of is
JEE Main 2022 (29 Jul Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Match List-I with List-II
| List-I | List-II | ||
| (A) | (I) | Amphoteric | |
| (B) | (II) | Basic | |
| (C) | (III) | Neutral | |
| (D) | (IV) | Acidic |
Choose the correct answer from the options given below
JEE Main 2022 (28 Jun Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
For electron gain enthalpies of the elements denoted as , the incorrect option is :
JEE Main 2023 (01 Feb Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
For compound having the formula , the correct option from the following is
JEE Main 2023 (11 Apr Shift 1)
Options
- A: Ga is coordinated with CI in
- B: Ga is more electronegative than AI and is present as a cationic part of the salt
- C: CI forms bond with both AI and Ga in
- D: Oxidation state of Ga in the salt
Explaination
Question
. Identify the correct order of standard enthalpy of formation of sodium halides.
JEE Main 2023 (13 Apr Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Bond dissociation energy of bond of the "" hydrides of group elements (given below), follows order.
(A)
(B)
(C)
(D)
JEE Main 2023 (30 Jan Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The correct order of first ionization enthalpy values of the following elements is : (A) \(\mathrm{O}\) (B) \(\mathrm{N}\) (C) \(\mathrm{Be}\) (D) \(\mathrm{F}\) (E) \(\mathrm{B}\) Choose the correct answer from the options given below :
JEE Main 2024 (04 Apr Shift 1)
Options
- A: E \( < \) C \( < \) A \( < \) B \( < \) D
- B: C \( < \) E \( < \) A \( < \) B \( < \) D
- C: B \( < \) D \( < \) C \( < \) E \( < \) A
- D: A \( < \) B \( < \) D \( < \) C \( < \) E
Explaination
Question
The correct order of the first ionization enthalpy is
JEE Main 2024 (04 Apr Shift 2)
Options
- A: \(\mathrm{Al} > \mathrm{Ga} > \mathrm{Tl}\)
- B: \(\mathrm{Ga} > \mathrm{Al} > \mathrm{B}\)
- C: \(\mathrm{Tl} > \mathrm{Ga} > \mathrm{Al}\)
- D: \(\mathrm{B} > \mathrm{Al} > \mathrm{Ga}\)
Explaination
Question
Match List I with List II \(\begin{array}{|l|l|c|l|} \hline & \text{List - I} & & \text{List - II} \\ \hline & \text{(Elements)} & & \text{(Properties in their respective groups)} \\ \hline \text { A. } & \mathrm{Cl}, \mathrm{S} & \text { I. } & \text { Elements with highest electronegativity } \\ \hline \text { B. } & \mathrm{Ge}, \mathrm{As} & \text { II. } & \text { Elements with largest atomic size } \\ \hline \text { C. } & \text { Fr, Ra } & \text { III. } & \begin{array}{l} \text { Elements which show properties of both } \\ \text { metals and non-metal } \end{array} \\ \hline \text { D. } & \text { F, O } & \text { IV. } & \begin{array}{l} \text { Elements with highest negative electron } \\ \text { gain enthalpy } \end{array} \\ \hline \end{array}\) Choose the correct answer from the options given below:
JEE Main 2024 (08 Apr Shift 1)
Options
- A: A-II, B-I, C-IV, D-III
- B: A-III, B-II, C-I, D-IV
- C: A-IV, B-III, C-II, D-I
- D: A-II, B-III, C-IV, D-I
Explaination
Question
Match List I with List II \(\begin{array}{|l|l|c|l|} \hline & \text{ List - I } & & \text{List - II} \\ \hline \text { A. } & \text { Melting Point }[\mathrm{K}] & \text { I. } & \mathrm{T} 1>\mathrm{In}>\mathrm{Ga}>\mathrm{A} 1>\mathrm{B} \\ \hline \text { B. } & \text { Ionic Radius }\left[\mathrm{M}^{+3} / \mathrm{pm}\right] & \text { II. } & \mathrm{B}>\mathrm{T} 1>\mathrm{A} 1 \approx \mathrm{Ga}>\mathrm{In} \\ \hline \text { C. } & \Delta_{\mathrm{i}} \mathrm{H}_1\left[\mathrm{~kJ} \mathrm{~mol}^{-1}\right] & \text { III. } & \mathrm{T} 1>\mathrm{In}>\mathrm{A} 1>\mathrm{Ga}>\mathrm{B} \\ \hline \text { D. } & \text { Atomic Radius }[\mathrm{pm}] & \text { IV. } & \mathrm{B}>\mathrm{A} 1>\mathrm{T} 1>\mathrm{In}>\mathrm{Ga} \\ \hline \end{array}\) Choose the correct answer from the options given below:
JEE Main 2024 (09 Apr Shift 2)
Options
- A: A-II, B-III, C-IV, D-I
- B: A-IV, B-I, C-II, D-III
- C: A-I, B-II, C-III, D-IV
- D: A-III, B-IV, C-I, D-II
Explaination
Question
The element having the highest first ionization enthalpy is
JEE Main 2024 (29 Jan Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Given below are two statements:
Statement - I: Along the period, the chemical reactivity of the element gradually increases from group to group .
Statement - II: The nature of oxides formed by group element is basic while that of group elements is acidic.
In the the light above statements, choose the most appropriate from the questions given below:
JEE Main 2024 (30 Jan Shift 2)
Options
- A: Both statement I and Statement II are true.
- B: Statement I is true but Statement II is False.
- C: Statement I is false but Statement II is true.
- D: Both Statement I and Statement II is false.
Explaination
Question
The correct sequence of electron gain enthalpy of the elements listed below is
A. Ar B. Br C. F D. S
Choose the most appropriate from the options given below:
JEE Main 2024 (31 Jan Shift 1)
Options
- A: C > B > D > A
- B: A > D > B > C
- C: A > D > C > B
- D: D > C > B > A
Explaination
Question
Consider the following elements.
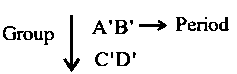
Which of the following is/are true about and ?
A. Order of atomic radii:
B. Order of metallic character :
C. Size of the element :
D. Order of ionic radii :
Choose the correct answer from the options given below:
JEE Main 2024 (31 Jan Shift 2)
Options
- A: A only
- B: A, B and D only
- C: A and B only
- D: B, C and D only
Explaination
Question
Given below are two statements :
Statement (I) : Both metal and non-metal exist in $\mathrm{p}$ and d-block elements.
Statement (II) : Non-metals have higher ionisation enthalpy and higher electronegativity than the metals.
In the light of the above statements, choose the most appropriate answer from the option given below:
JEE Main 2024 (01 Feb Shift 2)
Options
- A: Both Statement I and Statement II are false
- B: Statement I is false but Statement II is true
- C: Statement I is true but Statement II is false
- D: Both Statement I and Statement II are true