Top Previous Year Questions - Coordination Compounds
Question
Simplified absorption spectra of three complexes ((i) and (ii) and (iii)) of ion are provided below; their values are marked as and respectively. The correct match between the complexes and their values is:
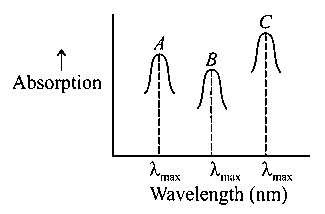
(i)
(ii)
(iii)
JEE Main 2020 (02 Sep Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The complex that can show optical activity is :
JEE Main 2020 (03 Sep Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The d-electron configuration of , and respectively are:
JEE Main 2020 (03 Sep Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The number of isomers possible for is:
JEE Main 2020 (04 Sep Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Consider the complex ions, trans- and The correct statement regarding them is :
JEE Main 2020 (05 Sep Shift 2)
Options
- A: both (A) and (B) cannot be optically active.
- B: (A) can be optically active, but (B) cannot be optically active.
- C: both (A) and (B) can be optically active.
- D: (A) cannot be optically active, but (B) can be optically active.
Explaination
Question
For a metal ion in an octahedral field, the correct electronic configuration is :
JEE Main 2020 (06 Sep Shift 2)
Options
- A: when
- B: when
- C: when
- D: when
Explaination
Question
The total number of coordination sites in ethylenediaminetetraacetate ( ) is ...................
JEE Main 2020 (05 Sep Shift 1)
Enter your answer
Explaination
Question
The Crystal Field Stabilization Energy (CFSE) and magnetic moment (spin-only) of an octahedral aqua complex of a metal ion are and , respectively. Identify
JEE Main 2021 (01 Sep Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Arrange the following metal complex/ compounds in the increasing order of spin only magnetic moment. Presume all the three, high spin system.
(Atomic numbers and .)
(a)
(b) and
(c)
JEE Main 2021 (16 Mar Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Match List-I with List-II :
| List-I | List-II | ||
| a | i | Linkage isomerism | |
| b | ii | Solvate isomerism | |
| c | iii | Co-ordination isomerism | |
| d | iv | Optical isomerism |
Choose the correct answer from the options given below:
JEE Main 2021 (17 Mar Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Spin only magnetic moment of an octahedral complex of in the presence of a strong field ligand in BM is:
JEE Main 2021 (20 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The calculated magnetic moments (spin only value) for species and respectively are:
JEE Main 2021 (24 Feb Shift 2)
Options
- A: and
- B: and
- C: and
- D: and
Explaination
Question
Which one of the following metal complexes is most stable?
JEE Main 2021 (25 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Arrange the following Cobalt complexes in the order of increasing Crystal Field Stabilization Energy (CFSE) value.
Complexes:
Choose the correct option :
JEE Main 2021 (26 Aug Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The additon of dilute to salt solution will give :
JEE Main 2021 (27 Aug Shift 2)
Options
- A: a solution of
-
B:
precipitate of
- C: precipitate of
- D: precipitate of
Explaination
Question
White precipitate of dissolves in aqueous ammonia solution due to formation of
JEE Main 2022 (25 Jun Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The metal complex that is diamagnetic is (Atomic number : )
JEE Main 2022 (26 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
cation gives a prussian blue precipitate on addition of potassium ferrocyanide solution due to the formation of
JEE Main 2022 (27 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Which of the following will have maximum stabilization due to crystal field?
JEE Main 2022 (27 Jun Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Match List-I with List-II
| List-I (Complex) | List-II (Hybridization) | ||
| A | I | ||
| B | II | ||
| C | III | ||
| D | IV |
Choose the correct answer from the options given below
JEE Main 2022 (28 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
In the cobalt-carbonyl complex : , number of bonds is "" and terminal ligands is " ". ___
JEE Main 2022 (24 Jun Shift 1)
Enter your answer
Explaination
Question
Consider the following metal complexes :
The spin-only magnetic moment value of the complex that absorbs light with shortest wavelength is B.M. (Nearest integer)
JEE Main 2022 (25 Jul Shift 1)
Enter your answer
Explaination
Question
The spin-only magnetic moment value of ion (in gaseous state) from the pairs and that has negative standard electrode potential, is_____
JEE Main 2022 (25 Jul Shift 2)
Enter your answer
Explaination
Question
If absorbs a light of wavelength for transition, then the value of octahedral crystal field splitting energy for will be____ [Nearest integer]
(Given : and )
JEE Main 2022 (25 Jun Shift 1)
Enter your answer
Explaination
Question
Match List I with List II
| List I Coordination entity | List II Wavelength of light absorbed in nm | ||
| A | I. | ||
| B | II. | ||
| C | III. | ||
| D | IV. |
Choose the correct answer from the options given below :-
JEE Main 2023 (25 Jan Shift 2)
Options
- A:
- B:
- C:
- D: