Top Previous Year Questions - General Organic Chemistry
Question
Which one of the following compounds is aromatic in nature?
JEE Main 2021 (01 Sep Shift 2)
Options
-
A:
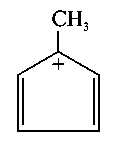
-
B:
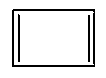
- C: Both A and B
-
D:
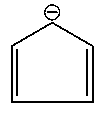
Explaination
Question
The correct pair(s) of the ambident nucleophiles is (are):
(A)
(B)
(C)
(D)
JEE Main 2021 (17 Mar Shift 2)
Options
- A: (B) and (C) only
- B: (A) only
- C: (A) and (C) only
- D: (B) only
Explaination
Question
Reagent, naphthylamine and sulphanilic acid in acetic acid is used for the detection of
JEE Main 2021 (18 Mar Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Given below are two statements :
Statement I : and both can generate nucleophile.
Statement II : and both will generate nitrile nucleophile with all reaction conditions.
Choose the most appropriate option :
JEE Main 2021 (18 Mar Shift 2)
Options
- A: Statement I is true but statement II is false
- B: Both statement I and statement II are true
- C: Statement I is false but statement II is true
- D: Both statement I and statement II are false
Explaination
Question
In Carius method, halogen containing organic compound is heated with fuming nitric acid in the presence of:
JEE Main 2021 (20 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Which purification technique is used for high boiling organic liquid compound (decomposes near its boiling point) ?
JEE Main 2021 (22 Jul Shift 1)
Options
- A: Simple distillation
- B: Steam distillation
- C: Fractional distillation
- D: Reduced pressure distillation
Explaination
Question
Which of the following molecules does not show stereo isomerism ?
JEE Main 2021 (22 Jul Shift 1)
Options
- A: -Dimethylhex--ene
- B: -Methylhex--ene
- C: -Ethylhex--ene
- D: -Methylhex--ene
Explaination
Question
Which one among the following resonating structures is not correct?
JEE Main 2021 (25 Jul Shift 1)
Options
-
A:
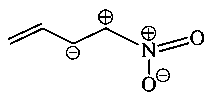
-
B:
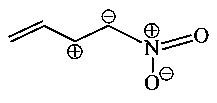
-
C:

-
D:
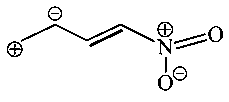
Explaination
Question
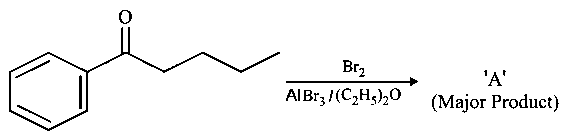
Consider the given reaction, the Product is:
JEE Main 2021 (26 Aug Shift 2)
Options
-
A:
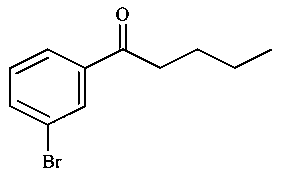
-
B:
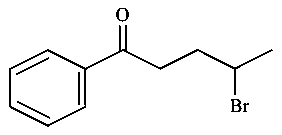
-
C:
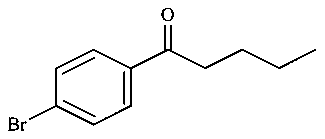
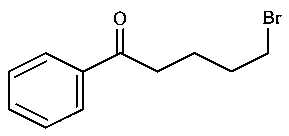
-
D:
Explaination
Question
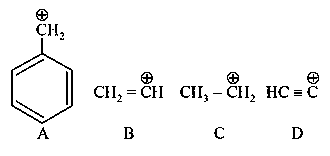
The correct order of stability of given carbocation is:
JEE Main 2021 (27 Jul Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Given below are two statements :
Statement I : Hyperconjugation is a permanent effect.
Statement II : Hyperconjugation in ethyl cation involves the overlapping of bond with empty orbital of other carbon.
Choose the correct option:
JEE Main 2021 (27 Jul Shift 2)
Options
- A: Both statement I and statement II are false
- B: Statement I is incorrect but statement II is true
- C: Statement I is correct but statement II is false
- D: Both Statement I and statement II are true.
Explaination
Question
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as
Assertion (A): A simple distillation can be used to separate a mixture of propanol and propanone.
Reason (R): Two liquids with a difference of more than in their boiling points can be separated by simple distillations.
In the light of the above statements, choose the most appropriate answer from the options given below :
JEE Main 2021 (31 Aug Shift 1)
Options
- A: Both (A) and (R) are correct but (R) is not the correct explanation of (A).
- B: Both (A) and (R) are correct and (R) is the correct explanation of (A).
- C: (A) is true but (R) is false.
- D: (A) is false but (R) is true.
Explaination
Question
Arrange the following conformational isomers of n-butane in order of their increasing potential energy: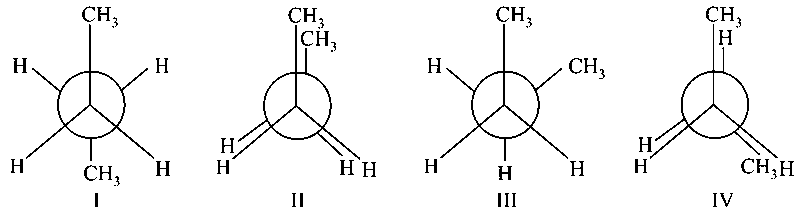
JEE Main 2021 (31 Aug Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The dihedral angle in staggered form of Newmann's projection of-Trichloro ethane is degree. (Round off to the nearest integer) (Round off to the nearest integer)
JEE Main 2021 (27 Jul Shift 2)
Enter your answer
Explaination
Question
Arrange the following in decreasing acidic strength.
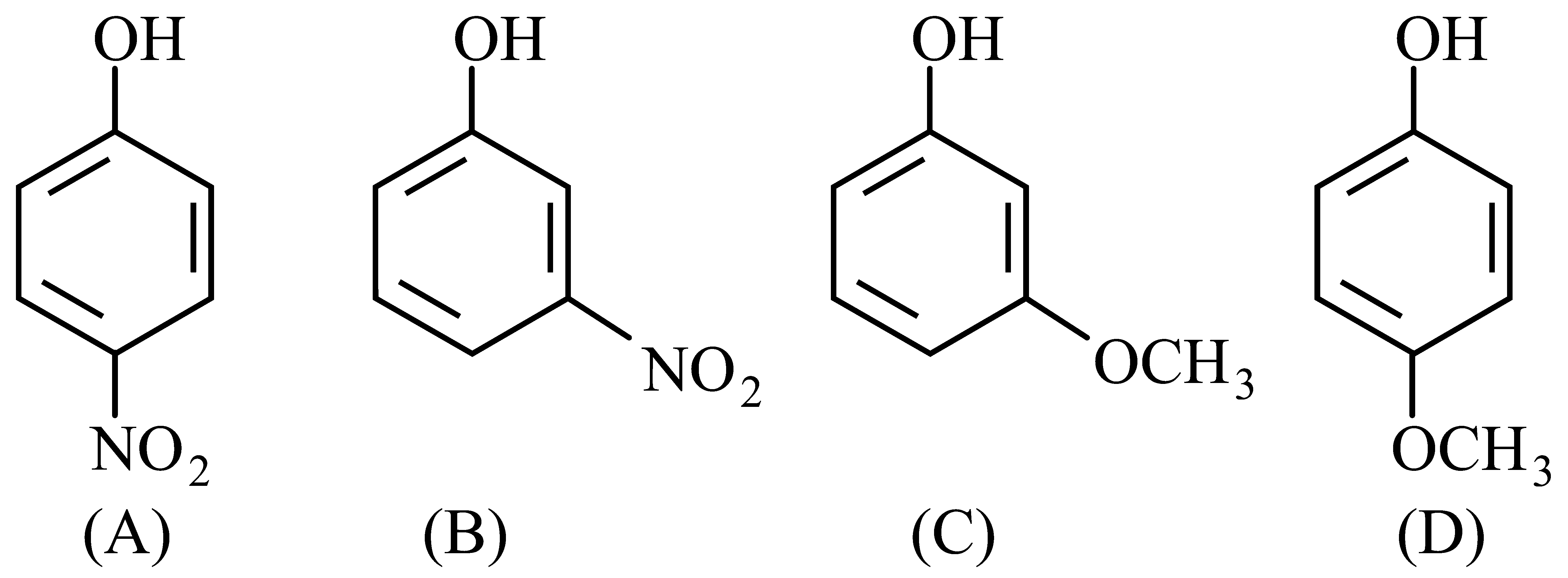
JEE Main 2022 (25 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
In the following structures, which one is having staggered conformation with maximum dihedral angle?
JEE Main 2022 (25 Jun Shift 1)
Options
-
A:
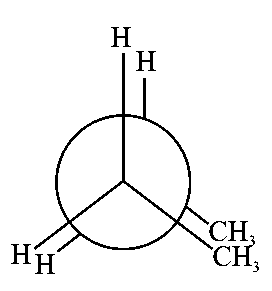
-
B:
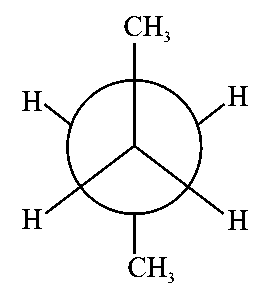
-
C:
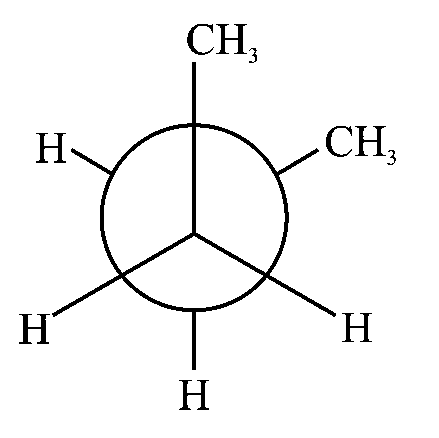
-
D:
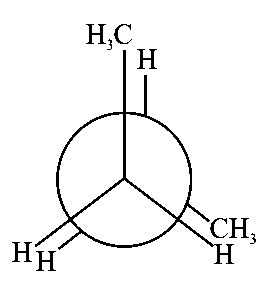
Explaination
Question
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason.
Assertion: A mixture contains benzoic acid and napthalene. The pure benzoic acid can be separated out by the use of benzene.
Reason: Benzoic acid is soluble in hot water.
In the light of the above statements, choose the most appropriate answer from the options given below.
JEE Main 2022 (25 Jun Shift 2)
Options
- A: Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
- B: Both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
- C: Assertion is true but Reason is false.
- D: Assertion is false but Reason is true.
Explaination
Question
Which of the following compounds is not aromatic?
JEE Main 2022 (26 Jul Shift 1)
Options
-
A:
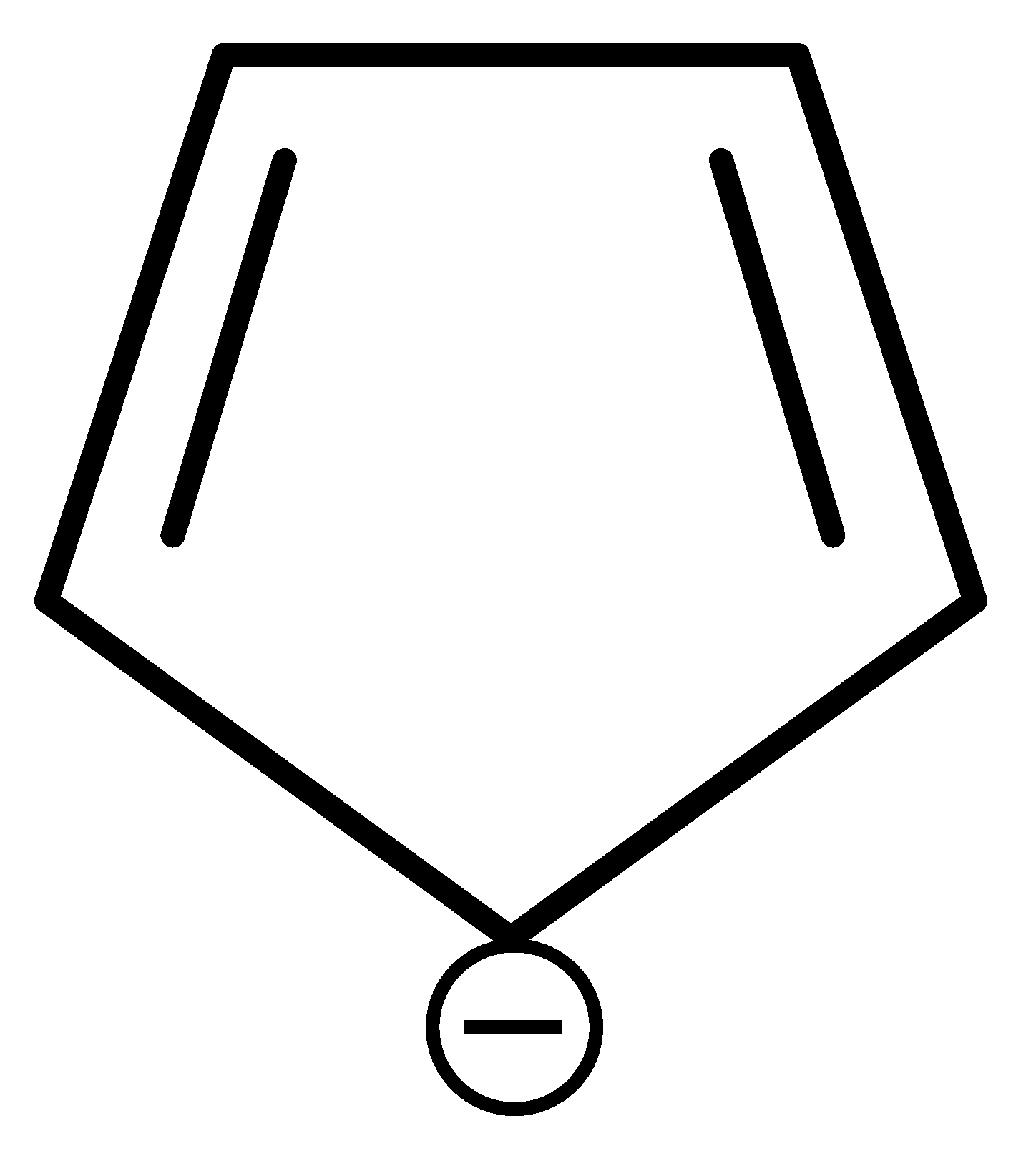
-
B:
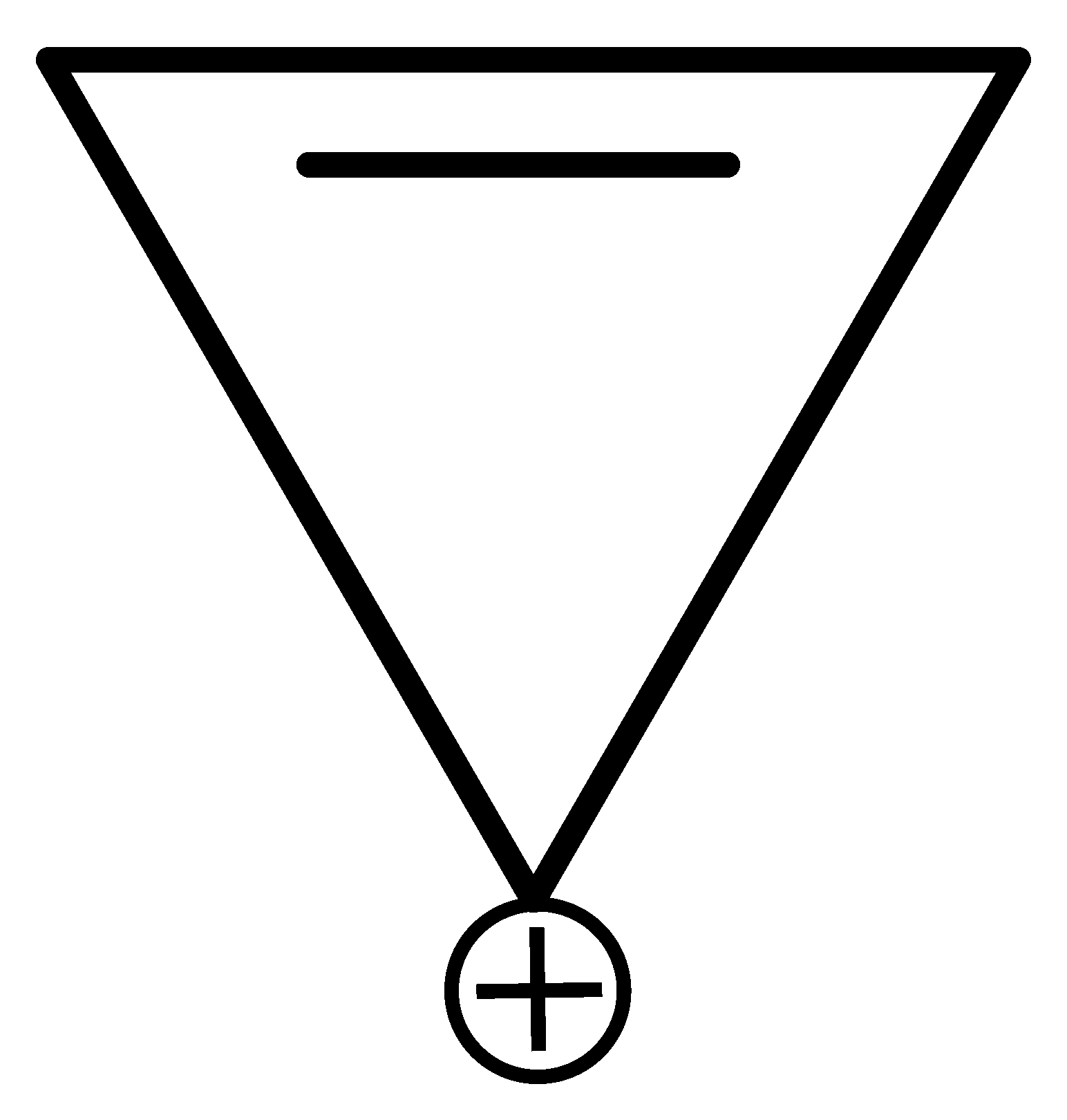
-
C:
-
D:
Explaination
Question
The correct stability order of the following diazonium salt is
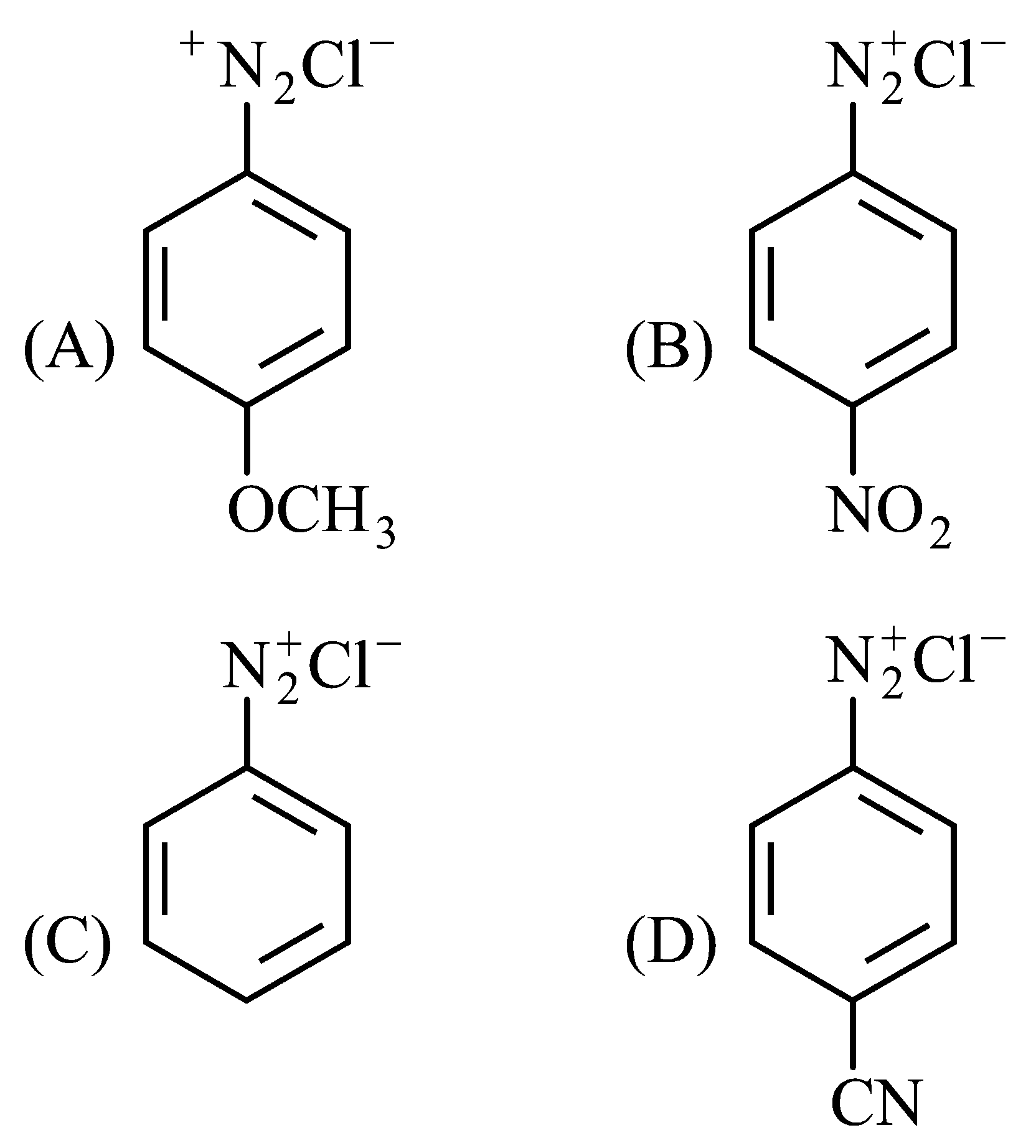
JEE Main 2022 (26 Jul Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
In Carius method of estimation of halogen. of an organic compound gave of . Find out the percentage of bromine in the compound.
(Molar masses : )
JEE Main 2022 (27 Jul Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Given below are two statements: One is labelled as Assertion and the other is labelled as Reason
Assertion : Thin layer chromatography is an adsorption chromatography.
Reason : A thin layer of silica gel is spread over a glass plate of suitable size in thin layer chromatography which acts as an adsorbent. In the light of the above statements, choose the correct answer from the options given below
JEE Main 2022 (28 Jul Shift 2)
Options
- A: Both and are true and is the correct explanation of
- B: Both and are true but is NOT the correct explanation of
- C: is true but is false
- D: is false but is true
Explaination
Question
Which of the following structures are aromatic in nature?
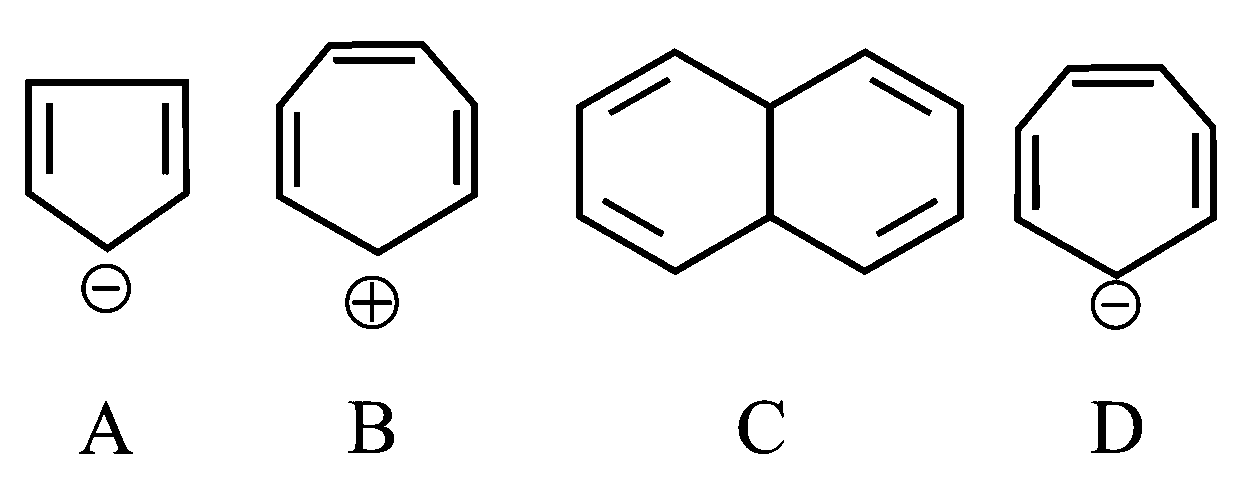
JEE Main 2022 (28 Jun Shift 1)
Options
- A:
- B: Only
- C: Only
- D: Only
Explaination
Question
The formula of the purple colour formed in Laissaigne's test for sulphur using sodium nitroprusside is
JEE Main 2022 (28 Jun Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The correct IUPAC name of the following compound is
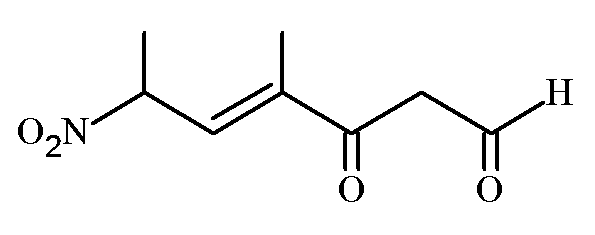
JEE Main 2022 (28 Jun Shift 2)
Options
- A: -methyl--nitro--oxohept--enal
- B: -methyl--oxo--nitrohept--enal
- C: -methyl--nitro--oxohept--enal
- D: -formyl--methyl--nitrohex--enal
Explaination
Question
Given below are two statements.
Statement I : The compound 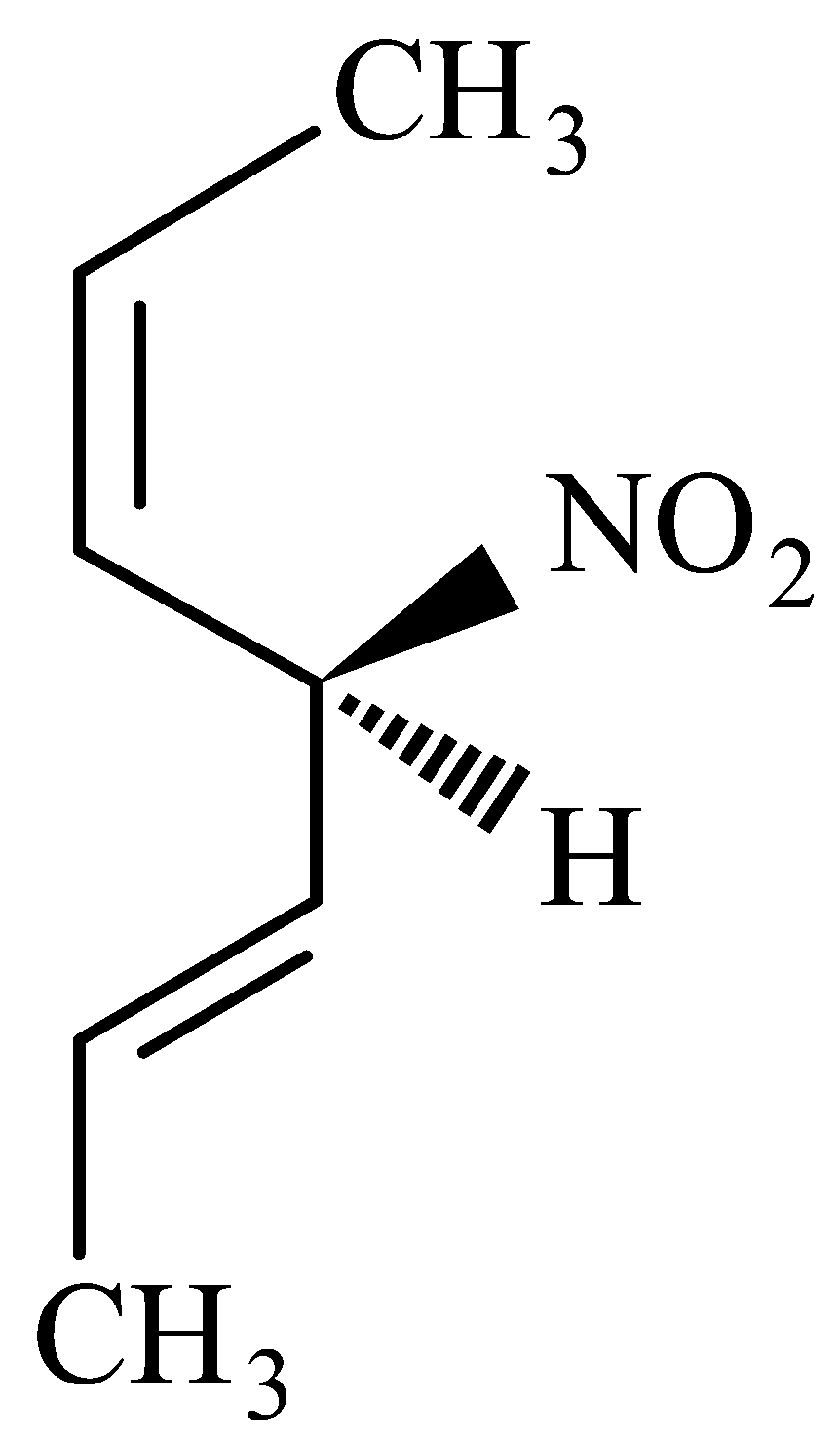 is optically active.
is optically active.
Statement II : 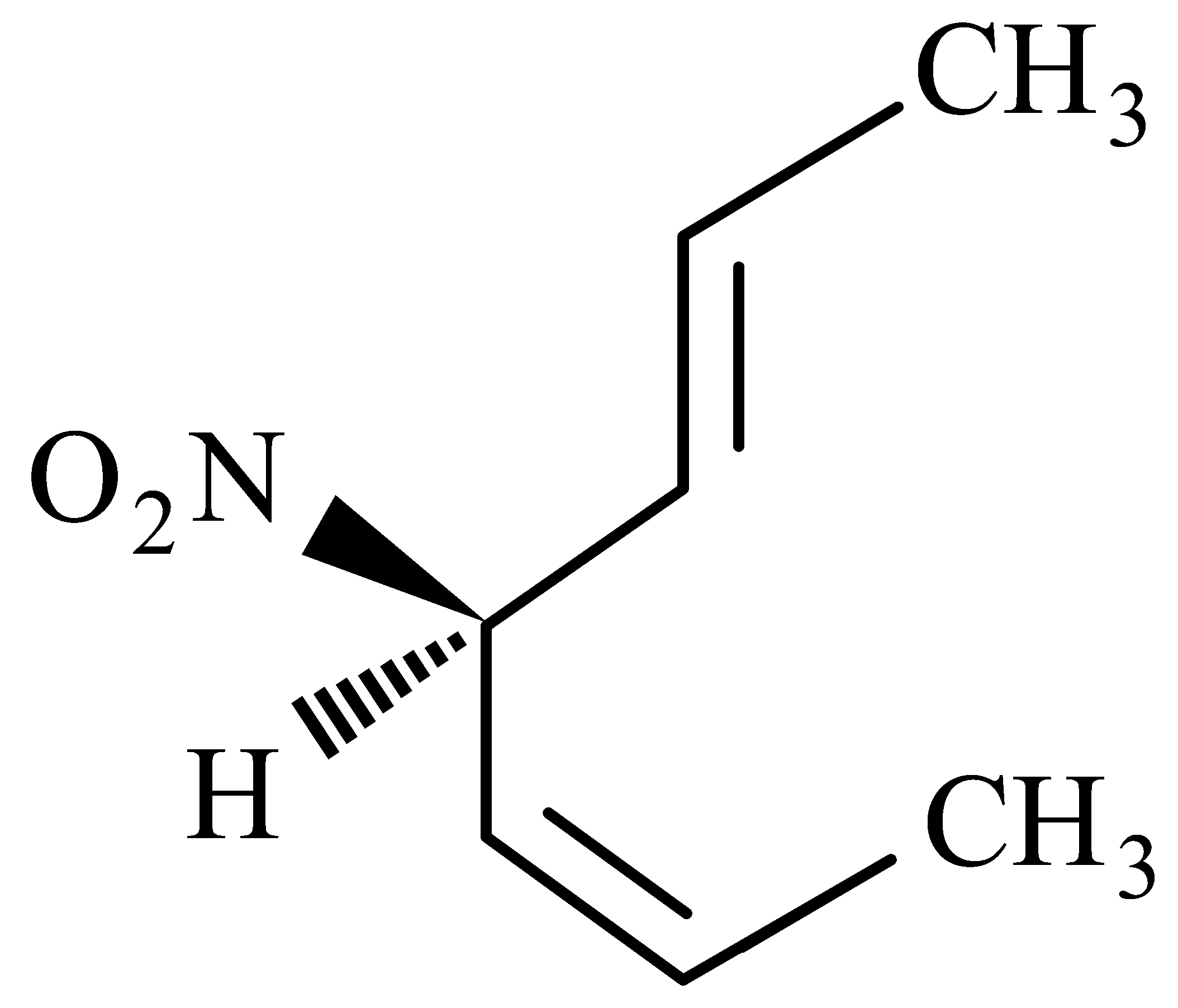 is mirror image of above compound .
is mirror image of above compound .
In the light of the above statement, choose the most appropriate answer from the options given below.
JEE Main 2022 (29 Jul Shift 2)
Options
- A: Both Statement I and Statement II are correct
- B: Both Statement I and Statement II are incorrect.
- C: Statement I is correct but Statement II is incorrect.
- D: Statement I is incorrect but Statement II is correct.