Top Previous Year Questions - Ionic Equilibrium
Question
The for the following dissociation is
Which of the following choices is correct for a mixture of and
JEE Main 2020 (09 Jan Shift 1)
Options
- A: Not enough data provided
- B:
- C:
- D:
Explaination
Question
A soft drink was bottled with a partial pressure of of 3 bar over the liquid at room temperature. The partial pressure of over the solution approaches a value of 30 bar when of is dissolved in of water at room temperature. The approximate of the soft drink is ___________.
(First dissociation constant of density of the soft drink )
JEE Main 2020 (05 Sep Shift 1)
Enter your answer
Explaination
Question
A solution is in and in
Solid is gradually added to it Assuming that the addition does not change in volume and and
Select correct statement from the following:
JEE Main 2021 (20 Jul Shift 2)
Options
- A: precipitates first because its is high.
- B: precipitates first as its is low.
- C: precipitates first because the amount of needed is low.
- D: will precipitate first as the amount of needed to precipitate is low.
Explaination
Question
moles of a weak acid is dissolved in of solution. The degree of dissociation of is __________ (Round off to the Nearest Integer). [Neglect volume change on adding and assume degree of dissociation ]
JEE Main 2021 (17 Mar Shift 1)
Enter your answer
Explaination
Question
of solution is titrated against solution. The following values were obtained in readings. and
Based on these readings, and convention of titrimetric estimation of concentration of solution is ___ .
(Round off to the Nearest integer)
JEE Main 2021 (18 Mar Shift 2)
Enter your answer
Explaination
Question
Assuming that is completely ionised in aqueous solution under the given conditions the concentration of ions in
aqueous solution of at is _________ (Nearest integer)
JEE Main 2021 (25 Jul Shift 2)
Enter your answer
Explaination
Question
is a sparingly soluble salt of molar mass and solubility The solubility product satisfies The value of is __________ . (Integer answer)
JEE Main 2021 (31 Aug Shift 1)
Enter your answer
Explaination
Question
The molar solubility of in solution is The value of is _____ . (Nearest integer)
JEE Main 2021 (01 Sep Shift 2)
Enter your answer
Explaination
Question
of is mixed with of . The of the mixture is nearest to:
(Given: , , )
JEE Main 2022 (25 Jul Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
and are the respective ionization constants for the following reactions and .
(a)
(b)
(c)
The relationship between and is given as
JEE Main 2022 (25 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The Plot of -metric titration of weak base vs strong acid looks like
JEE Main 2022 (27 Jul Shift 2)
Options
-
A:
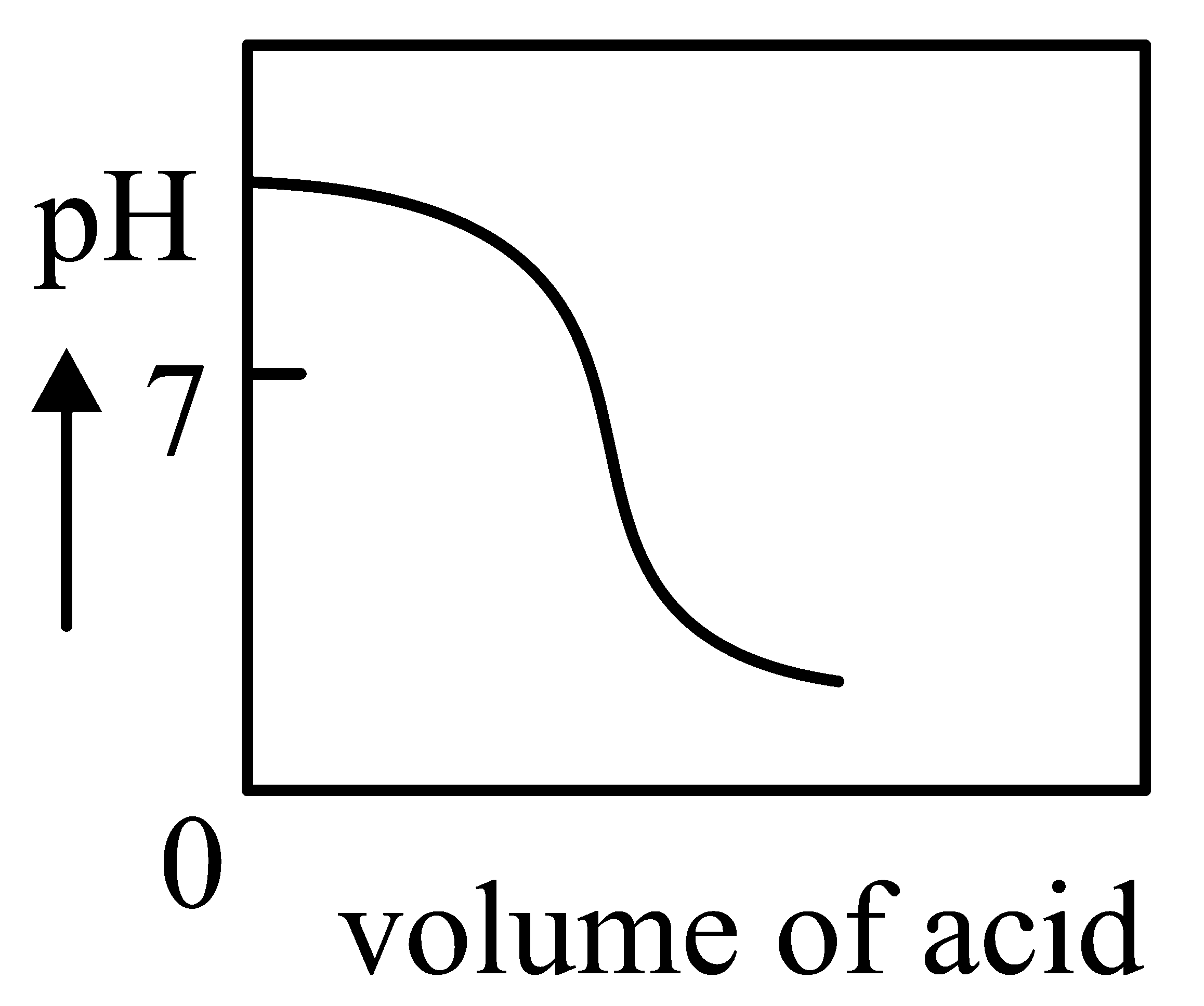
-
B:
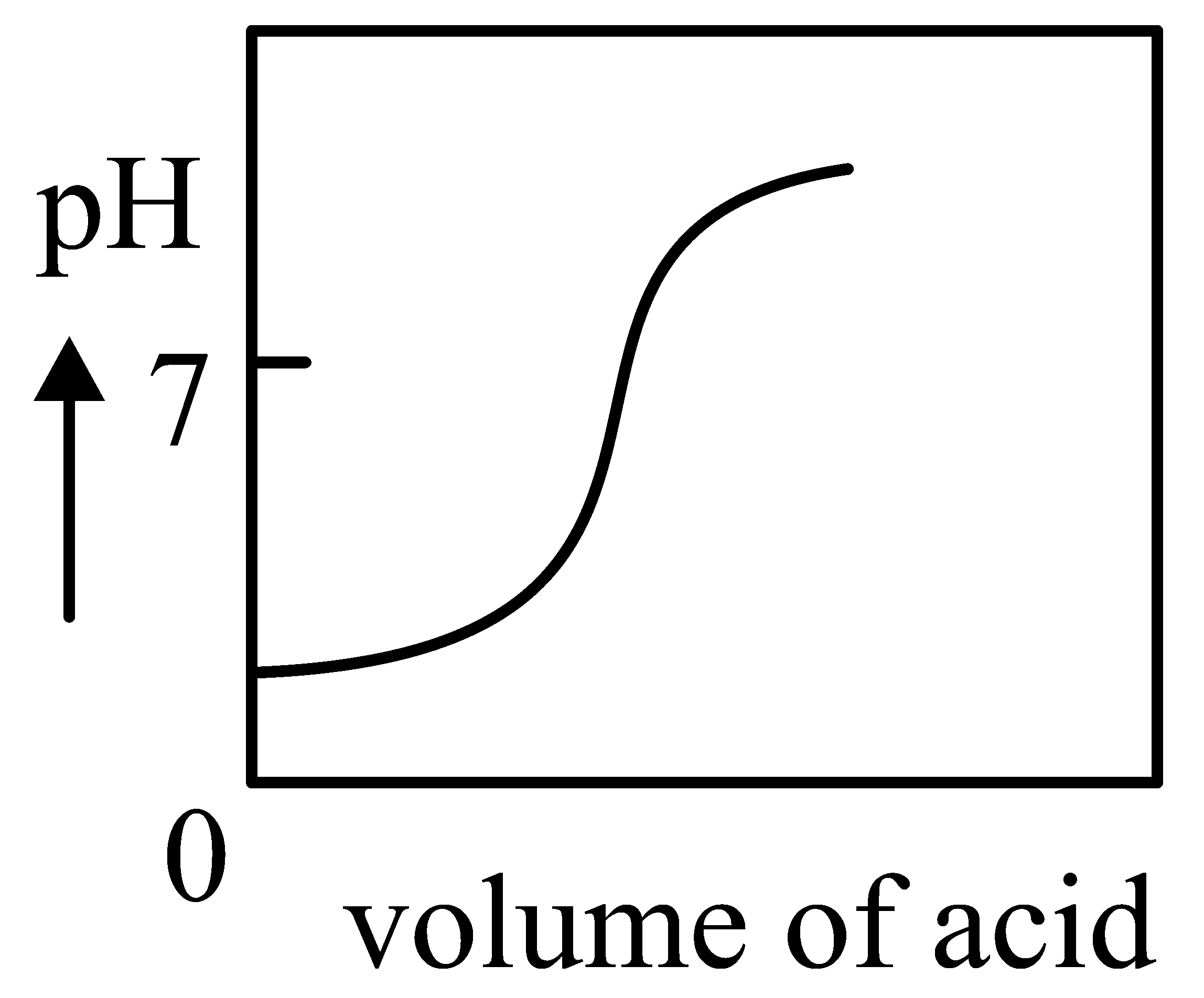
-
C:
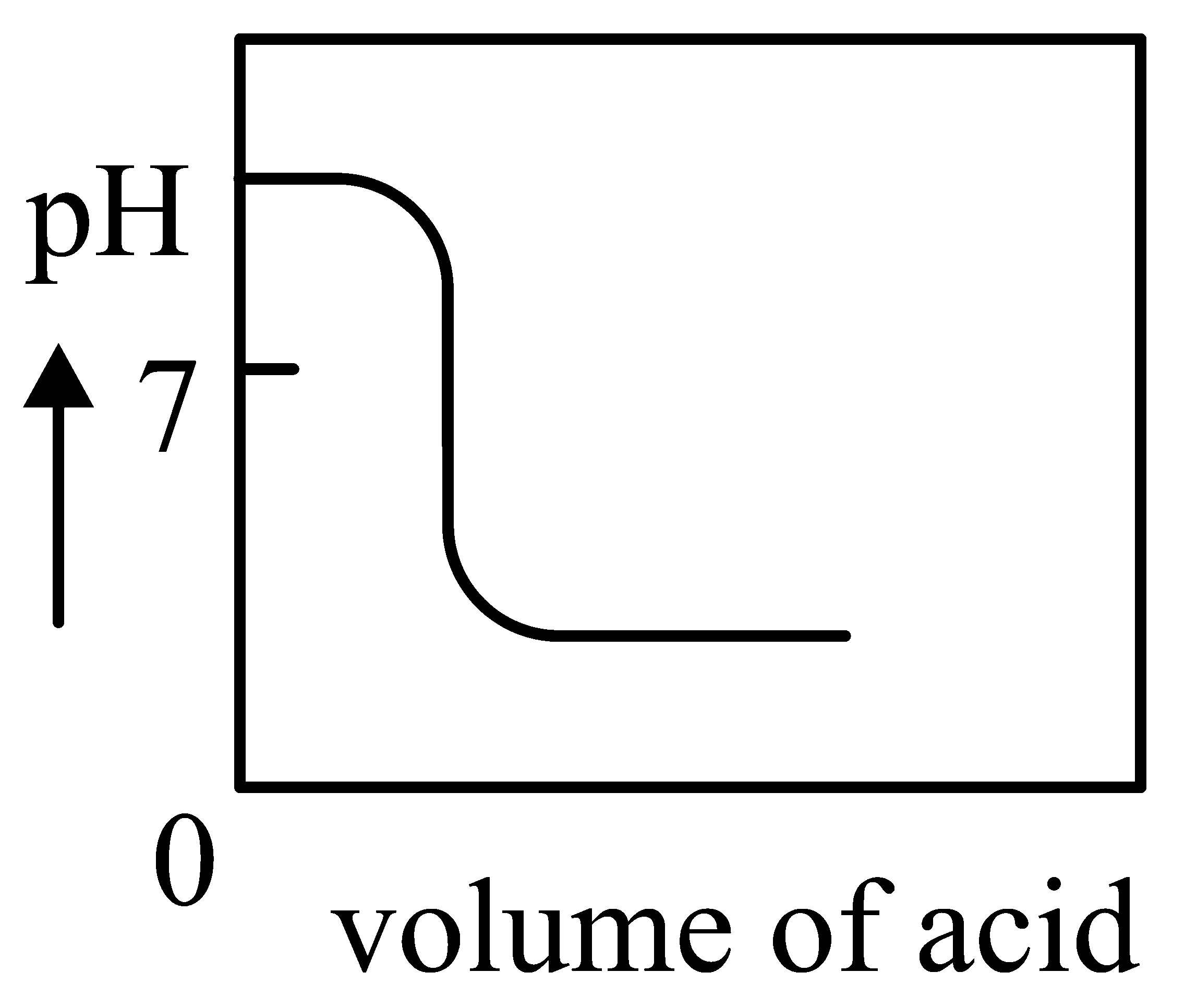
-
D:

Explaination
Question
of is mixed with of . The of the mixture is
JEE Main 2022 (29 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
of is being titrated against . When of has been added, the of the solution will be____. (Nearest integer)
(Given : )
JEE Main 2022 (26 Jun Shift 1)
Enter your answer
Explaination
Question
At , the solubility of in water is . The solubility product of is ---- (nearest integer). (Given molar mass : )
JEE Main 2022 (27 Jul Shift 1)
Enter your answer
Explaination
Question
The solubility of will be maximum in which of the following?
JEE Main 2022 (29 Jun Shift 1)
Options
- A:
- B:
- C: Deionised water
- D:
Explaination
Question
The dissociation constant of acetic is . When solution is mixed with solution, the of the resultant solution is found to be equal to . The value of is _______.
JEE Main 2023 (24 Jan Shift 1)
Enter your answer
Explaination
Question
of is added to of acetic acid solution. The of the resulting solution is . (Nearest integer) Given :