Top Previous Year Questions - Solutions
Question
Henry's constant (in kbar) for four gases and in water at is given below :
(density of water at ) This table implies that :
JEE Main 2020 (03 Sep Shift 1)
Options
- A: has the highest solubility in water at a given pressure
- B: solubility of at is lower than at
- C: The pressure of a molal solution of is bar
- D: The pressure of molal solution of is .
Explaination
Question
At the vapour pressure of is Hg and that of acetone is A solution of in acetone has a total vapour pressure of . The false statement amongst the following is:
JEE Main 2020 (07 Jan Shift 1)
Options
- A: Raoult's law is not obeyed by this system
- B: a mixture of and acetone has a volume
- C: and acetone are less attracted to each other than to themselves
- D: heat must be absorbed in order to produce the solution at
Explaination
Question
The elevation of boiling point of aqueous solution is two times that of aqueous solution. The value of is
[Assume ionisation of the complex and coordination number of as and that all molecules are present inside the coordination sphere]
JEE Main 2020 (06 Sep Shift 1)
Enter your answer
Explaination
Question
A cylinder containing an ideal gas ( of ) is in thermal equilibrium with a large volume of molal aqueous solution of ethylene glycol at its freezing point. If the stoppers and (as shown in the figure) are suddenly withdrawn, the volume of the gas in litres after equilibrium is achieved will be _____________.
(Given, 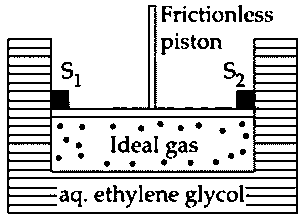
JEE Main 2020 (09 Jan Shift 2)
Enter your answer
Explaination
Question
molal solution of a weak acid has a freezing point of The degree of dissociation of this acid is ______ (Round off to the Nearest Integer). Given : Molal depression constant of water Freezing point of pure water
JEE Main 2021 (18 Mar Shift 1)
Enter your answer
Explaination
Question
gas is bubbled through water during a soft drink manufacturing process at . If exerts a partial pressure of then of would dissolve in of water. The value of is _______. (Nearest integer)
(Henry's law constant for at is )
JEE Main 2021 (25 Jul Shift 1)
Enter your answer
Explaination
Question
of solute A was dissolved in of ethanol and freezing point of the solution was found to be . The molar mass of solute is . [Given: Freezing point of ethanol is . Density of ethanol is . Freezing point depression constant of ethanol is ]
JEE Main 2022 (29 Jul Shift 2)
Enter your answer
Explaination
Question
Some amount of dichloromethane is added to of chloroform to prepare solution of . The concentration of is _____ ppm (by mass).
Given: Atomic mass : density of
JEE Main 2023 (30 Jan Shift 1)
Enter your answer
Explaination
Question
The Molarity \((\mathrm{M})\) of an aqueous solution containing \(5.85 \mathrm{~g}\) of NaCl in \(500 \mathrm{~mL}\) water is : (Given : Molar Mass \(\mathrm{Na}: 23\) and \(\mathrm{Cl}: 35.5 \mathrm{gmol}^{-1}\) )
JEE Main 2024 (04 Apr Shift 1)
Options
- A: 2
- B: 20
- C: 4
- D: 0.2
Explaination
Question
\(2.7 \mathrm{~kg}\) of each of water and acetic acid are mixed. The freezing point of the solution will be \(-x^{\circ} \mathrm{C}\). Consider the acetic acid does not dimerise in water, nor dissociates in water. \(x=\) ______ (nearest integer) [Given: Molar mass of water \(=18 \mathrm{~g} \mathrm{~mol}^{-1}\), acetic acid \(=60 \mathrm{~g} \mathrm{~mol}^{-1}\) \(\mathrm{K}_{\mathrm{f}} \mathrm{H}_2 \mathrm{O}: 1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\) \(\mathrm{K}_{\mathrm{f}}\) acetic acid: \(3.90 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\) freezing point: \(\mathrm{H}_2 \mathrm{O}=273 \mathrm{~K}\), acetic acid \(=290 \mathrm{~K}\)]
JEE Main 2024 (04 Apr Shift 2)
Enter your answer
Explaination
Question
The vapour pressure of pure benzene and methyl benzene at \(27^{\circ} \mathrm{C}\) is given as 80 Torr and 24 Torr, respectively. The mole fraction of methyl benzene in vapour phase, in equilibrium with an equimolar mixture of those two liquids (ideal solution) at the same temperature is _______ \(\times 10^{-2}\) (nearest integer)
JEE Main 2024 (09 Apr Shift 2)
Enter your answer
Explaination
Question
What happens to freezing point of benzene when small quantity of napthalene is added to benzene?
JEE Main 2024 (30 Jan Shift 1)
Options
- A: Increases
- B: Remains unchanged
- C: First decreases and then increases
- D: Decreases
Explaination
Question
Identify the mixture that shows positive deviations from Raoult's Law
JEE Main 2024 (31 Jan Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The osmotic pressure of a dilute solution is at . Osmotic pressure of the same solution at is .(Nearest integer)
JEE Main 2024 (29 Jan Shift 1)
Enter your answer
Explaination
Question
Given below are two statements: Statement I : Gallium is used in the manufacturing of thermometers. Statement II : A thermometer containing gallium is useful for measuring the freezing point \((256 \mathrm{~K})\) of brine solution. In the light of the above statements, choose the correct answer from the options given below :
JEE Main 2024 (06 Apr Shift 1)
Options
- A: Both Statement I and Statement II are true
- B: Statement I is false but Statement II is true
- C: Both Statement I and Statement II are false
- D: Statement I is true but Statement II is false