Top Previous Year Questions - Some Basic Concepts of Chemistry
Question
The strengths of volume hydrogen peroxide (of density ) in terms of mass percentage and molarity respectively, are: (Take molar mass of hydrogen peroxide as )
JEE Main 2020 (03 Sep Shift 2)
Options
- A: 1.7 and 0.5
- B: 0.85 and 0.25
- C: 1.7 and 0.25
- D: 0.85 and 0.5
Explaination
Question
A solution of two components containing moles of the component and moles of the component is prepared. and are the molecular weights of component 1 and 2 respectively. If is the density of the solution in is the molarity and is the mole fraction of the component, then can be expressed as :
JEE Main 2020 (06 Sep Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
molecules are present in of a substance The molarity of a solution containing of substance 'x' in 2 L solution is
JEE Main 2020 (03 Sep Shift 2)
Enter your answer
Explaination
Question
Complete combustion of of an organic compound provides of and of . The percentage composition of carbon and hydrogen in organic compound is and ______ respectively. (Round off to the Nearest Integer)
JEE Main 2021 (16 Mar Shift 1)
Enter your answer
Explaination
Question
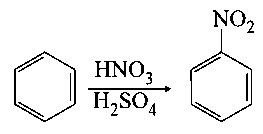
In the above reaction, of benzene on nitration gives of nitrobenzene. The percentage yield of nitrobenzene in the above reaction is _________ . (Round off to the Nearest Integer).
(Given atomic mass : )
JEE Main 2021 (17 Mar Shift 1)
Enter your answer
Explaination
Question
of compound was used to make of its aqueous solution. The molarity of the solution in is The value of is_____ (Rounded off to the nearest integer)
JEE Main 2021 (24 Feb Shift 1)
Enter your answer
Explaination
Question
The weighed out to make of an aqueous solution containing per is_ . (Rounded off to the nearest integer) [Given : Atomic weight in ]
JEE Main 2021 (26 Feb Shift 2)
Enter your answer
Explaination
Question
If a rocket runs on a fuel and liquid oxygen, the weight of oxygen required and released for every litre of fuel respectively are :
(Given : density of the fuel is )
JEE Main 2022 (24 Jun Shift 1)
Options
- A: and
- B: and
- C: and
- D: and
Explaination
Question
Hemoglobin contains of iron by mass. The number of atoms in of hemoglobin is (Given : Atomic mass of Fe is in)
JEE Main 2022 (26 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
A commercially sold conc. is by mass. If the density of this commercial acid is , the molarity of this solution is : (Atomic mass : )
JEE Main 2022 (26 Jun Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
solution of -glucose in water contains of carbon by weight. The molality of the solution is nearest to (Given: Atomic Weights are )
JEE Main 2022 (27 Jul Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Consider the reaction
The amount of required to produce of is
(Given : Atomic masses of and are and , respectively.)
JEE Main 2022 (29 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
When coal of purity is allowed to burn in presence of insufficient oxygen, of carbon is converted into ' ' and the remaining is converted into ''.
The heat generated when of coal is burnt is
JEE Main 2022 (29 Jul Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Production of iron in blast furnace follows the following equation
when of and of are allowed to react then the amount of iror (in ) produced is :
[Given: Molar Atomic mass
Molar Atomic mass
Molar Atomic mass ]
JEE Main 2022 (29 Jun Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
of nitrogen gas is mixed with excess of hydrogen gas and it is found that of ammonia gas is produced, The volume of unused nitrogen gas is found to be_____ .
JEE Main 2022 (25 Jul Shift 2)
Enter your answer
Explaination
Question
In the given reaction,
if one mole of each of and with of gives compound . (Given : Atomic masses of and are 10,20 and , respectively). The yield of is____.
JEE Main 2022 (28 Jul Shift 1)
Enter your answer
Explaination
Question
The density of ' \(x\) ' \(\mathrm{M}\) solution (' \(X\) ' molar) of \(\mathrm{NaOH}\) is \(1.12 \mathrm{~g} \mathrm{~mL}^{-1}\), while in molality, the concentration of the solution is \(3 \mathrm{~m}(3 \mathrm{molal})\). Then \(x\) is (Given : Molar mass of \(\mathrm{NaOH}\) is \(40 \mathrm{~g} / \mathrm{mol}\) )
JEE Main 2024 (06 Apr Shift 1)
Options
- A: 3.5
- B: 3.8
- C: 2.8
- D: 3.0
Explaination
Question
Molarity \((\mathrm{M})\) of an aqueous solution containing \(x \mathrm{~g}\) of anhyd. \(\mathrm{CuSO}_4\) in \(500 \mathrm{~mL}\) solution at \(32{ }^{\circ} \mathrm{C}\) is \(2 \times 10^{-1} \mathrm{M}\). Its molality will be ______ \(\times 10^{-3} \mathrm{~m}\). (nearest integer). [Given density of the solution \(=1.25 \mathrm{~g} / \mathrm{mL}\)]
JEE Main 2024 (09 Apr Shift 1)
Enter your answer
Explaination
Question
The molarity of orthophosphoric acid having purity by weight (specific gravity ) is ______ . (Molar mass of )
JEE Main 2024 (31 Jan Shift 2)
Enter your answer
Explaination
Question
of gaseous hydrocarbon on combustion gives of and of water vapour. Total number of carbon and hydrogen atoms in the hydrocarbon is _______.