Top Previous Year Questions - Thermodynamics (C)
Question
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.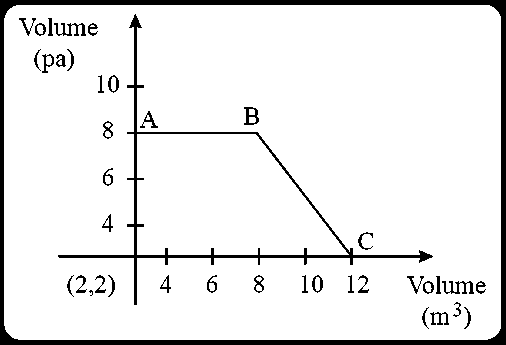
JEE Main 2020 (08 Jan Shift 1)
Enter your answer
Explaination
Question
For the reaction
the reaction enthalpy is
(Round off to the Nearest Integer).
[Given : Bond enthalpies in
JEE Main 2021 (18 Mar Shift 1)
Enter your answer
Explaination
Question
Five moles of an ideal gas at is expanded isothermally from an initial pressure of to against at constant external pressure . The heat transferred in this process is ___ . (Rounded-off to the nearest integer)
[ Use ]
JEE Main 2021 (25 Feb Shift 2)
Enter your answer
Explaination
Question
The equilibrium constant at for the reaction
is Starting with an equimolar solution with concentrations of and all equal to , the equilibrium concentration of is _____. (Nearest integer)
JEE Main 2021 (26 Aug Shift 2)
Enter your answer
Explaination
Question
When of solution is mixed with of solution, the increase in temperature of the final solution is . (Round off to the nearest integer).
[Use :
Specific heat of , density of
Assume no change in volume of solution on mixing.
JEE Main 2021 (27 Jul Shift 2)
Enter your answer
Explaination
Question
At and atm pressure, the enthalpies of combustion are as given below:
| Substance | (graphite) | ||
The enthalpy of formation of ethane is
JEE Main 2022 (24 Jun Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
Match List - I with List - II.
| List-I | List-II | ||
| Spontaneous process | |||
| Process with | |||
| Isothermal and isobaric process | |||
| Exothermic Process | [Bond energies of molecules in reactants] - [Bond energies of product molecules] |
Choose the correct answer from the options given below
JEE Main 2022 (27 Jun Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The enthalpy of combustion of propane, graphite and dihydrogen at are: , and respectively. The magnitude enthalpy of formation of propane is _____ . (Nearest integer)
JEE Main 2022 (25 Jul Shift 1)
Enter your answer
Explaination
Question
coal is burnt in a bomb calorimeter in excess of oxygen at and pressure.
The temperature of the calorimeter rises from to . The enthalpy change during the combustion of coal is . The value of is_____(Given : Heat capacity of bomb calorimeter . Assume coal to be pure carbon)
JEE Main 2022 (26 Jul Shift 1)
Enter your answer
Explaination
Question
A gas ( Molar mass ) was burnt in excess in a constant volume calorimeter and during combustion the temperature of calorimeter increased from to . If the heat capacity of calorimeter is and enthalpy of combustion of gas is then amount of gas burnt is____.
JEE Main 2022 (27 Jul Shift 2)
Enter your answer
Explaination
Question
When moles of gas expand isothermally and reversibly at from litre to litre, the magnitude of the maximum work obtained is . [nearest integer ] (Given : and )
JEE Main 2022 (27 Jun Shift 2)
Enter your answer
Explaination
Question
When of is mixed with of solution in a flask, the rise in temperature of the flask is_____
(Enthalpy of neutralisation and Specific heat of water ) (Neglect heat capacity of flask)
JEE Main 2022 (29 Jul Shift 1)
Enter your answer
Explaination
Question
of completely vapourises at and bar pressure and the enthalpy change in the process is . The enthalpy change for the vapourisation of of under the same conditions is .
JEE Main 2022 (29 Jun Shift 1)
Enter your answer
Explaination
Question
What happens when methane undergoes combustion in systems A and B respectively?
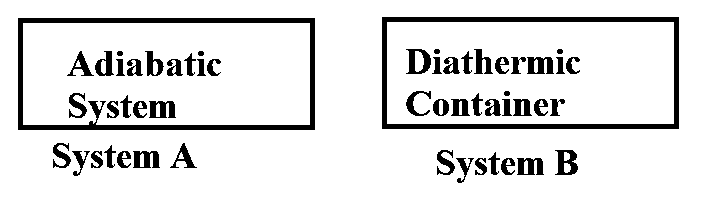
JEE Main 2023 (13 Apr Shift 2)
Options
-
A:
System A System B Temperature rises Temperature remains same -
B:
System A System B Temperature remains same Temperature rises -
C:
System A System B Temperature falls Temperature remains same -
D:
System A System B Temperature falls Temperature rises
Explaination
Question
L of is produced on complete combustion of gaseous mixture of ethene and methane at and atm. Heat evolved during the combustion process is
Given
.
JEE Main 2023 (25 Jan Shift 2)
Enter your answer
Explaination
Question
The enthalpy of formation of ethane \(\left(\mathrm{C}_2 \mathrm{H}_6\right)\) from ethylene by addition of hydrogen where the bond-energies of \(\mathrm{C}-\mathrm{H}, \mathrm{C}-\mathrm{C}, \mathrm{C}=\mathrm{C}, \mathrm{H}-\mathrm{H}\) are \(414 \mathrm{~kJ}, 347 \mathrm{~kJ}, 615 \mathrm{~kJ}\) and \(435 \mathrm{~kJ}\) respectively is \(\qquad\) \(\mathrm{kJ}\)
JEE Main 2024 (04 Apr Shift 1)
Enter your answer
Explaination
Question
Combustion of 1 mole of benzene is expressed at \(\mathrm{C}_6 \mathrm{H}_6(\mathrm{l})+\frac{15}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow 6 \mathrm{CO}_2(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \text {. }\) The standard enthalpy of combustion of \(2 \mathrm{~mol}\) of benzene is \(-^{\prime} x^{\prime} \mathrm{kJ}\). \(x=\) ______ Given: 1. standard Enthalpy of formation of \(1 \mathrm{~mol}\) of \(\mathrm{C}_6 \mathrm{H}_6(\mathrm{l})\), for the reaction \(6 \mathrm{C}\) (graphite) \(+3 \mathrm{H}_2(\mathrm{~g}) \rightarrow \mathrm{C}_6 \mathrm{H}_6(\mathrm{l})\) is \(48.5 \mathrm{~kJ} \mathrm{~mol}^{-1}\). 2. Standard Enthalpy of formation of \(1 \mathrm{~mol}\) of \(\mathrm{CO}_2(\mathrm{~g})\), for the reaction \(\mathrm{C}\) (graphite) \(+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})\) is \(-393.5 \mathrm{~kJ} \mathrm{~mol}^{-1}\). 3. Standard and Enthalpy of formation of \(1 \mathrm{~mol}\) of \(\mathrm{H}_2 \mathrm{O}(\mathrm{l})\), for the reaction \(\mathrm{H}_2(\mathrm{~g})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{l})\) is \(-286 \mathrm{~kJ} \mathrm{~mol}^{-1}\).
JEE Main 2024 (05 Apr Shift 2)
Enter your answer
Explaination
Question
An ideal gas, \(\overline{\mathrm{C}}_{\mathrm{v}}=\frac{5}{2} \mathrm{R}\), is expanded adiabatically against a constant pressure of 1 atm untill it doubles in volume. If the initial temperature and pressure is \(298 \mathrm{~K}\) and \(5 \mathrm{~atm}\), respectively then the final temperature is _______ \(\mathrm{K}\) (nearest integer). [\(\overline{\mathrm{C}}_{\mathrm{v}}\) is the molar heat capacity at constant volume]
JEE Main 2024 (06 Apr Shift 1)
Enter your answer
Explaination
Question
If three moles of an ideal gas at expand isothermally from to against a constant opposing pressure of , then the amount of heat transferred is __________J.
JEE Main 2024 (27 Jan Shift 1)
Enter your answer
Explaination
Question
If moles of an ideal gas expands from to a volume of at under isothermal and reversible condition then work, , is . The value of is _______.
(Given )