Top Previous Year Questions - Thermodynamics
Question
Three different processes that can occur in an ideal monoatomic gas are shown in the vs diagram. The paths are labelled as and . The change in internal energies during these process are taken as and and the work done as and . The correct relation between these parameters are:
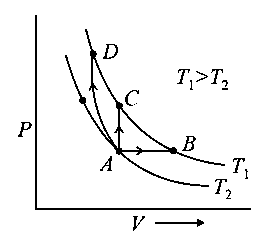
JEE Main 2020 (05 Sep Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
A thermodynamic cycle is shown on a - diagram.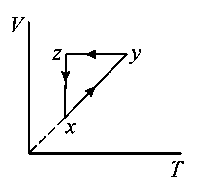
The - diagram that best describes this cycle is: (Diagrams are schematic and not to scale)
JEE Main 2020 (08 Jan Shift 1)
Options
-
A:

-
B:
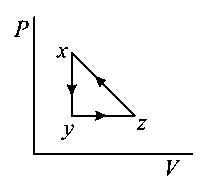
-
C:
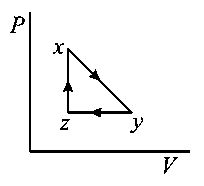
-
D:
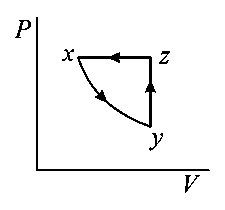
Explaination
Question
Starting at temperature one mole of an ideal diatomic gas is first compressed adiabatically from volume to It is then allowed to expand isobarically to volume . If all the processes are the quasi-static then the final temperature of the gas (in ) is (to the nearest integer) ___________.
JEE Main 2020 (09 Jan Shift 2)
Enter your answer
Explaination
Question
Which one is the correct option for the two different thermodynamic processes ?
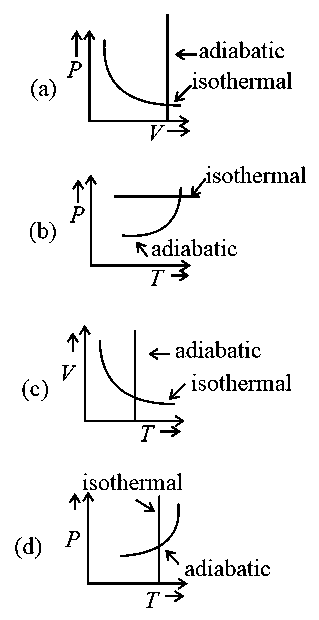
JEE Main 2021 (17 Mar Shift 2)
Options
- A: (c) and (a)
- B: (c) and (d)
- C: (a) only
- D: (b) and (c)
Explaination
Question
Which of the following graphs represent the behaviour of an ideal gas? Symbols have their usual meaning.
JEE Main 2021 (20 Jul Shift 2)
Options
-
A:
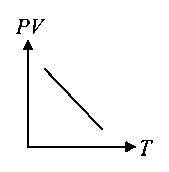
-
B:
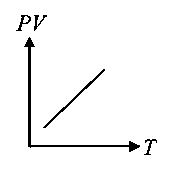
-
C:
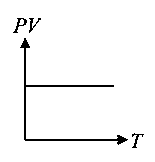
-
D:
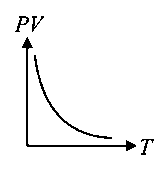
Explaination
Question
If one mole of an ideal gas at is allowed to expand reversibly and isothermally ( to ) its pressure is reduced to one-half of the original pressure (see figure). This is followed by a constant volume cooling till its pressure is reduced to one-fourth of the initial value Then it is restored to its initial state by a reversible adiabatic compression ( to ). The net workdone by the gas is equal to:
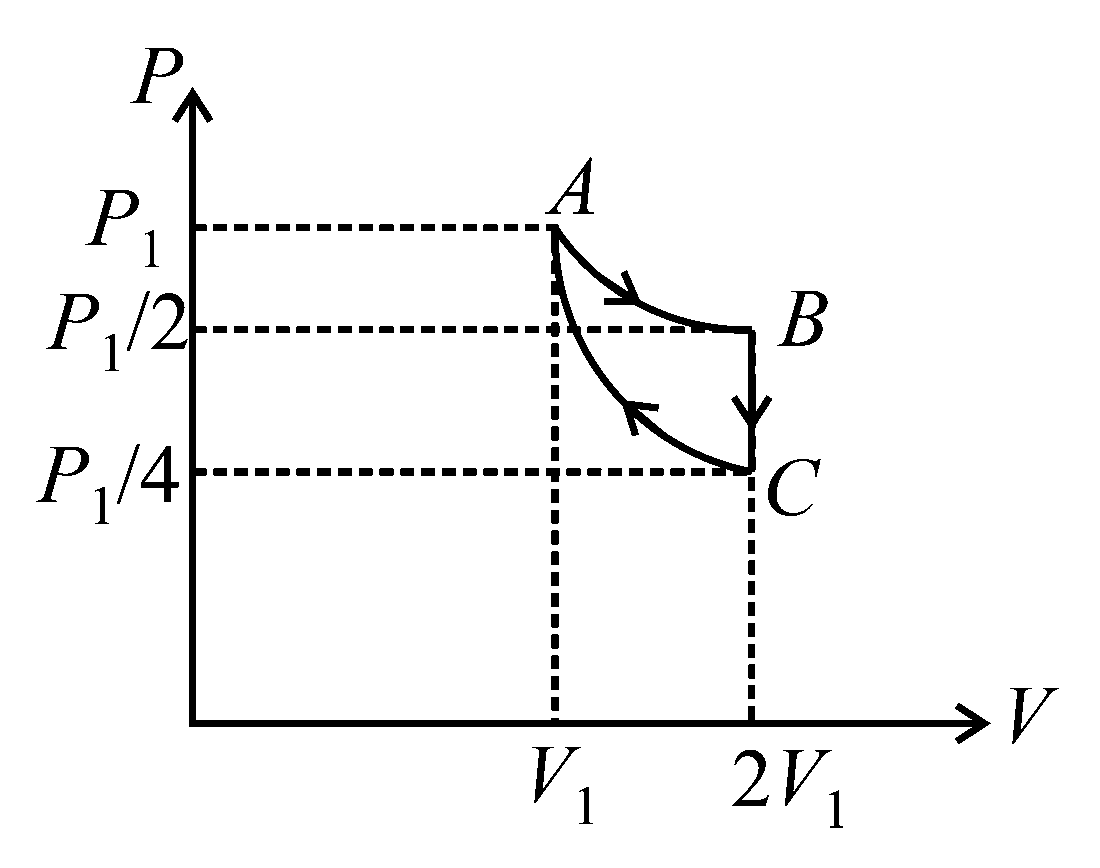
JEE Main 2021 (24 Feb Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
One mole of an ideal gas is taken through an adiabatic process where the temperature rises from to . If the ideal gas is composed of polyatomic molecule that has 4 vibrational modes, which of the following is true?
JEE Main 2021 (27 Jul Shift 2)
Options
- A: work done by the gas is close to
- B: work done on the gas is close to
- C: work done by the gas is close to
- D: work done on the gas is close to
Explaination
Question
One mole of an ideal gas at is taken from to as shown in the given indicator diagram. The work done by the system will be _______ [Given, ] (Round off to the nearest integer)
(Round off to the nearest integer)
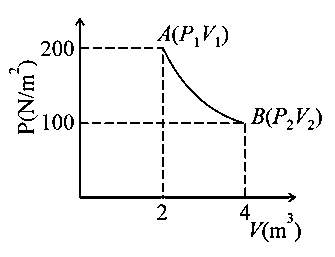
JEE Main 2021 (20 Jul Shift 2)
Enter your answer
Explaination
Question
A certain amount of gas of volume at temperature and pressure expands isothermally until its volume gets doubled. Later it expands adiabatically until its volume gets redoubled. The final pressure of the gas will be (Use )
JEE Main 2022 (25 Jul Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
At a certain temperature, the degrees of freedom per molecule for gas is . The gas performs of work when it expands under constant pressure. The amount of heat absorbed by the gas will be _____ .
JEE Main 2022 (28 Jul Shift 2)
Enter your answer
Explaination
Question
In an Isothermal change, the change in pressure and volume of a gas can be represented for three different temperature; as:
JEE Main 2023 (24 Jan Shift 2)
Options
-
A:
-
B:
-
C:
-
D:
Explaination
Question
Let be the ratio of molar specific heat at constant pressure and molar specific heat at constant volume of a monoatomic gas and be the similar ratio of diatomic gas. Considering the diatomic gas molecule as a rigid rotator, the ratio is:
JEE Main 2023 (24 Jan Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
A hypothetical gas expands adiabatically such that its volume changes from litres to litres. If the ratio of final pressure of the gas to initial pressure of the gas is . Then the ratio of will be.
JEE Main 2023 (31 Jan Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
P-T diagram of an ideal gas having three different densities $\rho_1, \rho_2, \rho_3$ (in three different cases) is shown in the figure. Which of the following is correct :
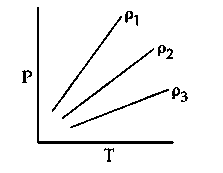
JEE Main 2024 (04 Apr Shift 1)
Options
- A: $\rho_1>\rho_2$
- B: $\rho_2 < \rho_3$
- C: $\rho_1=\rho_2=\rho_3$
- D: $\rho_1 < \rho_2$
Explaination
Question
A sample of 1 mole gas at temperature $\mathrm{T}$ is adiabatically expanded to double its volume. If adiabatic constant for the gas is $\gamma=\frac{3}{2}$, then the work done by the gas in the process is:
JEE Main 2024 (09 Apr Shift 1)
Options
- A: $\frac{R}{T}[2-\sqrt{2}]$
- B: $\frac{T}{R}[2+\sqrt{2}]$
- C: RT $[2-\sqrt{2}]$
- D: $\mathrm{RT}[2+\sqrt{2}]$
Explaination
Question
A real gas within a closed chamber at $27^{\circ} \mathrm{C}$ undergoes the cyclic process as shown in figure. The gas obeys $P V^3=R T$ equation for the path $A$ to $B$. The net work done in the complete cycle is (assuming $R=8 \mathrm{~J} / \mathrm{mol} \mathrm{K}$ ):
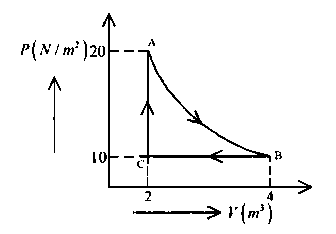
JEE Main 2024 (09 Apr Shift 2)
Options
- A: $20 \mathrm{~J}$
- B: $205 \mathrm{~J}$
- C: $-20 \mathrm{~J}$
- D: $225 \mathrm{~J}$
Explaination
Question
A thermodynamic system is taken from an original state to an intermediate state by a linear process as shown in the figure. Its volume is then reduced to the original value from to by an isobaric process. The total work done by the gas from to and to would be :
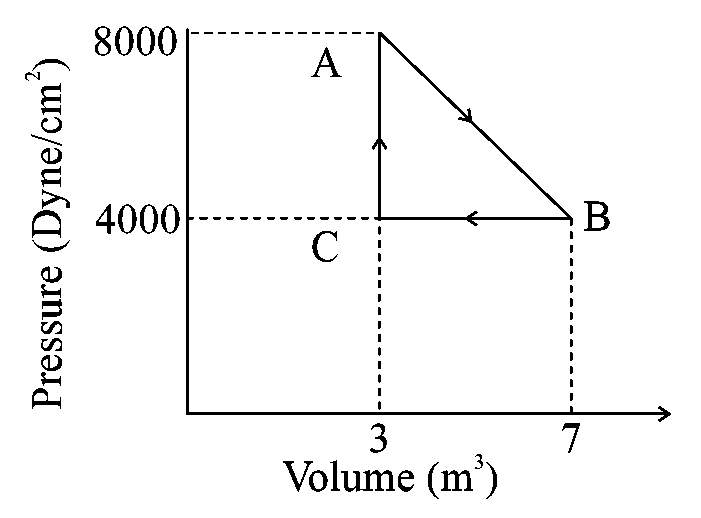
JEE Main 2024 (29 Jan Shift 1)
Options
- A:
- B:
- C:
- D:
Explaination
Question
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio of for the gas is :
JEE Main 2024 (27 Jan Shift 2)
Options
- A:
- B:
- C:
- D:
Explaination
Question
The given figure represents two isobaric processes for the same mass of an ideal gas, then
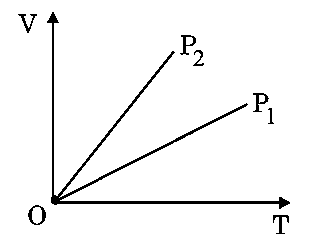
JEE Main 2024 (31 Jan Shift 1)
Options
- A:
- B:
- C:
- D: